FAQs
Why do we need a testable hypothesis?
We need a hypothesis towards discovering the mechanism of operation of the nervous system that provides internal sensations of various higher brain functions such as perception, memory and consciousness. Without knowing this, we will not be able to understand the system. In this context, hypothesis development is essential to understand how internal sensations are induced in a system with nearly 1011 neurons and 1015 synapses. A single counter example of proof against a hypothesis can then be used as sufficient reason to modify or reject it. According to Karl Popper, a philosopher of science, a hypothesis must be falsifiable; i.e. it must at least in principle be possible to make an observation that would disprove the proposition as false, even if one has not actually (yet) made that observation (Popper, 1965). Once such an observation is made, it will lead to rejection of the hypothesis. However, even with the rejection of a hypothesis, we are likely to make some conclusions that will aid in the development of new and better hypotheses.
The nervous system is being studied by several faculties of sciences at various levels – biochemical, cellular, electrophysiological, systems, behavioural, imaging. In order to explain all these features, the solution must be a unique one. In this regard, a testable hypothesis is highly valuable. Even though the internal sensations cannot be directly examined, we can circumvent the difficulty. If a simple unique solution can be derived to explain all the findings made at various levels, then this solution must be right (This is similar to viewing an unknown variable in an equation within a solvable system of linear equations where the values of all the other variables are known. Please see this example). This motivated to develop a hypothesis for nervous system functions. The hypothesis can then be verified in biological systems by a) postdictive examination of several previous findings to test whether they can be explained by the hypothesized mechanism, b) searching for the predictions that can be made from the hypothesis, and b) examining comparable circuit features for different sensations in remote species of animals. Once verified, it can be further studied by the gold standard test of replicating the mechanism in engineered systems. This approach will truly enable us to undertake a cost-effective research work in the right direction.
Why should we study the formation of first-person inner sensations? Can't we understand the brain without studying it?
Each organ in the body is formed to execute very specific functions. For example, heart is formed to pump blood to other organs. Each and every cell in the heart is associated with this function, one way or other. We can't think of studying the heart by ignoring its pumping action. Similarly we can't think of studying the kidneys by ignoring their filtering action. If we look at the brain, its most important function is generation of first-person inner sensations of different brain functions such as perception, memory, and thought process. It also executes motor actions based on the survival needs determined by the first-person inner sensations of decision making. Due to this reason, we can't study the brain by ignoring its unique & most important function of generation of inner sensations that we call "mind." Wiring of the brain is evolved to generate robust first-person inner sensations within it, which is essential for the very survival of all the animals. We have not been paying attention to the probable locations and mechanism by which it is generated, most likely due to our lack of confidence in discovering methods to verify them. When we ignore pathways that generate first-person inner sensations, we are ignoring the major circuit connections that are responsible for their generation.
What function should we begin examining to build the hypothesis?
Learning and memory are the best functions to study the nervous system operations. This is because we can 1) induce changes in the nervous system during associative learning that can be verified, 2) induce first-person internal sensation of retrieved memories in physiological time-scales, 3) carry out loss of function studies, 4) test whether the hypothesis can be extended to understand consolidation of memories, perception and consciousness, 5) replicate in engineered systems to test for the formation of the first-person inner sensation of memory, and 6) use the very large amount of already collected data to verify the hypothesis being built at its various stages. For example, the following questions can be addressed. a) What parallel cellular changes are taking place during testing for long-term potentiation (LTP) with a regular stimulus and retrieval of memories? b) How LTP can get correlated with the surrogate markers of behavioural motor activities indicative of the induction of internal sensation of memory?
How does artificial intelligence community see first-person properties recently?
Members of AI community have started wondering how neuroscientists approch towards providing a mechanistic explanation for cognition. They want neurosciene to figure out the mechanism by which brain generates its functions. For example read: Neurotechnology is Critical for AI Alignment (cvitkovic.net)
What is the difference between single synapse strengthening hypothesis and semblance hypothesis?
From the Hebb’s postulates, it was derived that synaptic plasticity changes the strengths of single synapses during learning. According to this postulate, if two stimuli are associatively learned, then the synapses along their paths are expected to undergo plasticity changes. However, it is not yet known how the arrival of one of the stimuli (cue stimulus) that propagates through its path utilizes the changes in synaptic strength to induce memory of the associatively learned second item. All the studies of synaptic plasticity changes occurring at the time of learning rely on animal behavior at the time of memory retrieval. It is difficult to interconnect synaptic plasticity changes with behavior and derive a mechanistic explanation for memory. In other words, until now it was not possible to find an explanation that will allow us to replicate the postulated mechanism in an engineered system. It is important to note that Hebb's postulates have guided our research until now, which has provided a very large number of observations. However, difficulties in obtaining a mechanistic explanation for the first-person internal sensation of memory from learning-induced changes that can be replicated in engineered systems prompt us to re-examine the Hebb's postulates, identify its drawbacks and formulate a new postulate. In this context, the present hypothesis was developed by asking the question, "At the time of memory retrieval, when one of the sensory stimuli (the cue stimulus) propagates through its path, how can it induce an inner sensation of memory of the associatively learned sensory stimulus (that moved through a second path at the time of learning) and also generate behavioral motor activity reminiscent of the associatively learned second stimulus?"
Based on the semblance hypothesis, when an associative learning takes place between two sensory stimuli, there should be certain changes at the locations where these stimuli converge (for example, hippocampus in spatial memory or amygdala in fear memory). This hypothesis examined the interaction between the synapses of the associatively learned stimuli at locations of their convergence. At a later time, when one of the stimuli (cue stimulus) arrives at the locations of convergence of the two sensory stimuli, the cue stimulus should be able to induce internal sensation of the memory of the associatively learned second stimulus within the physiological time-scales of milliseconds. Therefore, semblance hypothesis focused on identifying the locus of interaction between the two neuronal pathways and more specifically, the sub-synaptic locations that belong to these two pathways between which learning induces certain changes from which the cue stimulus can induce inner sensations of memory of the second stimulus. In this approach, it is not possible to use neuronal firing due to several reasons explained in the answer to the next question.
What are the limitations of studying neuronal firing (somatic spike) in understanding higher brain functions?
Studies using behavior as a surrogate marker
for memory have detected firing of specific sets of neurons during both
learning and memory retrieval. For example, compared to the set of
neurons that fire when exposed to one of the associatively learned
stimuli (cue stimulus) before learning, additional neurons are fired
when an animal is exposed to the same cue stimulus after learning. This
is documented in the lateral amygdala in fear conditioning experiments (Schoenbaum
et al., 1998; Tye et al., 2008).
Manipulation of neuronal firing also resulted
in identification of firing of specific sets of neurons during learning
& memory retrieval (Tonegawa et
al., 2015; Josselyn and
Tonegawa, 2020). However, neural
network studies being carried out for more than fifty years are finding
severe difficulties in solving the nervous system. When we find a
replicable mecahnism of learning that can be used to generate memories,
it is hoped that we will be able to explain how ensembles of neurons
fire during learning and memory retrieval. In the light of necessity to
understand memories in their true nature as first-person inner
sensations, examination of conditions under which a neuron fire shows
the following findings that need urgent consideration.
1) Investigations during the last 15 years have shown that in addition to axonal spikes (neuronal firing or action potential), there are spiking potentials occurring at the dendrites (Antic et al., 2010; Moore et al., 2017). Spikes are the instantaneous summaton (summing up) of potentials occurring in a localized region. The purpose of the axonal spike is to propagate the potentials towards all the axonal terminals of the neurons. However, we have to still discover the function of the dendritic spikes. Only by directing our studies to interconnect as many observations as possible, we will be able to find the functional attributes of dendritic spikes that will help us to solve the system.
2) The number of input connections (postsynapses or postsynaptic terminals or dendritic spines) varies widely among the neurons. It ranges from one (passive conductance of potentials between the initial orders of neurons of the visual pathway) to approximately 5,600 (as in a monkey’s visual cortex) to 60,000 (as in a monkey’s motor cortex) (Cragg 1967). Most often, the arrival of a tiny fraction of inputs is sufficient to fire a neuron. Several earlier experiments provided hints that spatial summation of nealry 40 inputs arriving at neuronal soma can generate an action potential (neuronal firing). Recent modelling studies have shown that a pyramidal neuron that has tens of thousands of input connections can fire an action potential by spatial summation (summation at the same time) of nearly 140 EPSPs at the axonal hillock that arrives from randomly located dendritic spines (Palmer et al. 2014; Eyal et al., 2018) (Note that it is possible to have nearly 40 to 50 EPSPs of high strength originating close to the soma that can fire a neuron. For further discussions, the number 140 will be used). Please note that temporal summation of even less than 140 EPSPs can induce an action potential. The combinatorial probability of the number of sets of synapses whose activation can give rise to the firing of a neuron is enormously high. This makes an action potential non-specific with regards to its inputs.
3) Thirdly, postsynaptic potentials contributing to both sub- and supra-threshold activation of a neuron do not contribute to the neuronal firing. Therefore, if there are mechanisms for inducing internal sensations occurring at the unaccounted synapses, they will get ignored if neuronal firing alone is examined. For example, let us take one pyramidal neuron (excitatory neuron) with 25,000 inputs (dendritic spines). If 3600 inputs (dendritic spines) are activated simultaneously (due to their synaptic activation) during an action, only one action potential will be elicited. A simultaneous arrival of 140 inputs at the axonal hillock is enough to induce that action potential. This means (3600 - 140) = 3460 EPSPs get wasted without having any functional use. Is this advantageous to the system? For the purpose of this discussion, let us assume that 140 EPSPs can fire a neuron. In this context, any set of inputs of less than 140 EPSPs that do not lead to the generation of the action potential is also getting wasted. In what context evolution would have conserved this mechanism? The input redundancy may be a possible mechanism to achieve a common set of outputs for operating the limited set of combinations of muscles in the body for achieving behavioral activities to survive in the environment. When a cue stimulus activity propagates to one of the inputs to a neuron at its sub- or supra-threshold activation state and if it does not lead to change in the non-firing/firing state of that neuron, can the information from the cue stimulus still be utilized by the system? In the context that we are still searching for a mechanism of induction of first-person internal sensation of memory, reminiscent of the sensory features of the learned item in its absence, it is necessary to examine a possible mechanism that occurs at the input level.
4) Postsynaptic potentials induced at the dendritic spines located at remote locations on the dendritic tree (for example, pyramidal neurons with long apical dendritic tree) have to travel long distances to reach towards the axon hillock to summate above the threshold for triggering the action potential. They degrade significantly as they reach the axon hillock (Spruston 2008). Therefore, the contributions of these potentials to neuronal firing get reduced and vary depending on the distance they have to travel and the dendritic diameter. This naturally leads to the question "Why would the observed degradation of potentials get conserved?" It is very likely that they are providing functions independent of the neuronal firing except in conditions where they contribute to the nth EPSP necessary to trigger an action potential. Whenever a postsynaptic potential degrades (attenuate), information is gradually lost. Therefore, we have to think about a mechanism other than neuronal firing. For preventing loss of information, it is necessary to have an operational mechanism taking place close to the origin of inputs (postsynaptic potentials), which will not be affected by the attenuation of postsynaptic potentials. This will provide an efficient mechanism whereby all the specific inputs can contribute to generate a specific brain function (e.g. specificity of memory).
5) Since EPSPs get degraded as the distance from the dendritic spine to the soma increases, in reality EPSPs from nearly 140 dendritic spines will get summated to fire a neuron. Let us assume that this pyramidal neuron has 10,000 dendritic spines (inputs or postsynaptic terminals). If EPSPs arriving from nearly 140 of its dendritic spines can fire that neuron, then nearly [1x104! ÷ (140! x (1x104! – 140!))] ≈ 2.79x10318 sets of combinations of input signals can fire that neuron. If we consider that a pyramidal neuron has only 3,000 dendritic spines, then the set of combinations will reduce to 1.72x10244 (To compare, note that the number of atoms in the observable Universe is only nearly 1082). Note that above calculations are done only for a fixed number of 140 input signals. When the number of input signals varies from 141 to 10,000 or 141 to 3000 respectively, each possibility needs separate calculations to find the number of possible combinations. Therefore, the sum of all the possible combinations will be a huge number. This means that a gigantic number of combinations of input signals can cause the same neuronal firing. Therefore, when we see a neuron firing (axonal spike) (in vivo, at physiological conditions), it is not at all specific with respect to its inputs. Understanding the extreme degeneracy of sets of input signals in firing a neuron is of paramount importance in making correlations between the firing of specific neurons (both natural and artificial) and those higher brain functions having unique internal sensations.
6) Many times, several neurons are held at subthreshold activation. It means that they will be receiving less than 140 postsynaptic potentials all the time, just short of few potentials for triggering an action potential (neuronal firing). Neurons located at higher orders than those that are firing in an oscillating fashion (reasons for these oscillating type of neuronal firing need explanation, especially the horizontal component of the oscillations – which are explained by the present hypothesis) are mostly held at a range of subthreshold values. For example, 138 or 139 inputs arriving at higher order neurons will not lead to the firing of those neurons. These sub-threshold-activated neurons require only 1 or 2 input signals to cause their firing. Therefore, when we see these neurons firing, these neuronal firings have to be interpreted completely differently.
All the above findings show that studies using neuronal firing and networks of firing-neurons do not examine specific mechanisms that are likely to take place at the level of the inputs (dendritic spines). In addition, when it comes to the need for explaining the first-person internal sensations of higher brain functions, current studies examining the third-person observations are a dimension away (third-person v/s first-person) from where we need to reach.
So what does a neuronal firing mean with respect to its inputs? From the above paragraphs, we have seen examples of conditions in which a neuron held at its baseline state can get fired by either 3600 inputs or just 1 input. In what context evolution would have conserved this mechanism? It may be a possible mechanism to achieve a common set of outputs for operating the limited set of combinations of muscles in the body for achieving behavioral activities to survive in the environment. In the context that we are still searching for a mechanism of induction of first-person internal sensations, reminiscent of that are induced by the external stimuli (in the latter's absence), it is required to examine possible mechanisms occurring at the input level. In the context of input redundancy in firing a neuron, this will avoid ignoring any valuable operational mechanism occurring at the input level. This will allow us to address the question from the previous subtitle "Where is the ideal location for convergence to occur that will allow the cue stimulus to induce internal sensation of the associatively learned second stimulus?" without ignoring the specificity of inputs brought by the cue stimulus. It is reasonable to expect interactive changes occurring at the input levels of the neurons at locations of convergence of associatively learned stimuli. This is examined in the new hypothesis.
Inner sensations cannot be accessed by third-person observers. Then, how can we study them?
To study things that our sensory systems do not have any access, we need to use the principles of the methods used in physics to study particles and fields to which also we do not have any access. The basic principle is based on the deep principle used in mathematics when finding a solution to a system of linear equations having a unique solution. It is the constraints provided by the terms in the equations that guide towards solving the system. In this approach, we need to use all the equations within the system to find that solution. Similarly, by using constraints from all the findings from various levels of the nervous system (see Table 2 on the first page of this website), it is possible to derive a solution for the system that induce units of internal sensations and integrate/process them at physiological time-scales. Once such a solution can be derived, then several postdictive findings can be examined for the validity of the solution. Once this stage succeeds, then predictions can be made that can be verified. This is a standard procedure used by physics to make discoveries. A similar approach can be undertaken to understand the location and mechanism of generation of units of internal sensations. The present work has followed these steps towards understanding the operational mechanism.
How can the information from fMRI studies be used to understand the operational mechanism?
From the Table 1 on the first page of this website, it can be seen that one of the requirements of the operating mechanism is that it should take place at physiological time scales. Since blood oxygenation level dependent (BOLD) signals initiate very slowly and take nearly 4 seconds to peak following a higher brain function or neural activity at the same location (Fig.2 in Monti et al., 2010; Figs.2-5 in Murayama et al., 2010), it does not provide information regarding the normal operational mechanism. However, when the actual mechanism of operation is known, it should be able to provide an explanation why oxygen is released at those locations following a time delay. In other words, the hypothesized mechanism should be able to accommodate a proper explanation for the BOLD signals.
What are the current challenges in memory research and how can we overcome them?
Memories are virtual internal sensations at the time of memory retrieval. The behavioral motor activities observed along with it should be considered as surrogate markers indicative of memory retrieval. Strong correlation between the experimental finding of long term potentiation (LTP) and the surrogate behavioral motor activities at the time of memory retrieval have been observed. However, alone, LTP has certain limitations. LTP takes at least 20 to 30 seconds (Gustafsson and Wigström, 1990) and even more than a minute to reach it's peak level of induction, which does not match with the physiological time-scales of changes occurring during associative learning. LTP was reported as lacking sufficiency to be the mechanism of memory storage (Shors and Matzel, 1997; Martin et al., 2000; Piorazi and Mel, 2001). Furthermore, several reported correspondences of LTP temporal phases do not correspond with that of memory phases (Abbas et al., 2015). In spite of these, the correlation between the behavioral markers of memory with LTP (excluding the time-scale issues) has some hidden facts that can provide a valuable piece of the puzzle towards understanding the cellular changes occurring during associative learning. In this context, it becomes necessary that the true mechanism of formation of first-person internal sensation of retrieved memories should be able to explain how LTP is related to memory.
Challenges in understanding the mechanistic changes during associative learning that enables cue-induced internal sensation of retrieved memory and its related effects on the observations in the field of psychology have been discussed (Gallistel and Balsam, 2014; Edelman, 2012). The challenges become manageable when it become possible to figure out a method to enter into the first-person frame of reference using third-person observed findings.
What are the general requirements for a theory of memory?
It should theoretically be able to explain the following features.
- Ability to learn at physiological time-scales (in milliseconds), following which memory can be retrieved.
- Retrieval of memory at physiological time-scales (in milliseconds).
- Provision for unlimited memory lifetimes (Rubin and Fusi, 2007).
- Ease of learning a related task.
- Disuse reduction in memory.
- Instant access to very large memory stores (Abbott, 2008).
- Should have provision for a mechanism for retaining specificity for retrieving memory.
- Functional integration of new neurons generated in the hippocampus.
- How basic units of memory from different learning events are used in a transferable manner (Dahlin et al., 2008).
- Ability to explain observed correlations between LTP & behavioral motor activities indicative of the formation of inner sensation of memory
- Explain observations of retrieval of memories of what was learned prior to 8 -10 years ago, following removal of hippocampi.
- Ability to explain the internal sensation of perception at least as a framework.
- Ability to explain the internal sensation of consciousness at least as a framework.
- Mechanism within the system to generate hypothesis (Abbott, 2008).
- Ability to explain some of the features of mental disorders (as a "loss of function" of normal operational mechanism.
A hypothesis that can provide a broad framework incorporating all the above features needs to be built and tested theoretically followed by experimental approaches to confirm the basic structural changes taking place both during associative learning and memory retrieval. The operating mechanism should take place within the synaptically-connected neuronal circuitry.
How long does it take for the learning mechanism to occur?
Humans have an ability to associate more than one pair of sensory stimuli in a rapid-fire exercise during a limited period of one second. The learning changes induced by more than one pair of associative learning stimuli can then be used to retrieve their corresponding memories. This indicates that learning mechanism can be "completed" in sub-second (milliseconds) duration. Depending on the duration for which learning-indued changes persist, memory can be retrieved at different durations following learning. More details in a Preprint.
What is the single most necessary step to succeed?
Associative learning induces changes in milliseconds (physiological time-scale). Using these changes (following learning), a cue stimulus can retrieve first-person internal sensation of memory in milliseconds. If the changes induced during the milliseconds of time during learning can remain within the system, then it should be able to provide the ability to retrieve memory after a long period of time, which we call as long-term memory. In this context, we must focus on changes occurring within millisecond time-scales at the time of learning. This should be the focus on understanding the science behind the operational functions of the nervous system. All the delayed molecular changes following learning can only have secondary effects on the primary change occurring during the milliseconds of time during learning.
How can constraints guide towards the solution?
How can
we
reach the solution using
a
large number of constraints provided
by findings from different levels of nervous system functions? This
approach is motivated by the methods used in physics to understand
particles and fields that we cannot sense directly using our
sensory systems. The deep underlying principle of this is based on
methods used in linear algebra for solving a system of large set of
linear equations that has a unique solution. Here, the relationships
between the variables in the equations guide us towards the solution. In
mathematics, it is possible to find quick methods to arrive at
the solution. In fact, we invent those quick methods. The natural
question at this point is that mathematics can develop
the
equations. Neuroscience is different. Yes, in mathematics all the
derivations can be carried out even without any equations. Equations
were invented by us so that others can derive the results of similar problems very quickly.
Always the first person who
invent such short cuts need to spend lots of time to design it
(In fact, students who study only the equations do not understand the
concept behind the process and they will not like mathematics. Once one
understands the process behind an equation, one will enjoy it and most
likely go for graduate studies in mathematics!). So
the point here is that if we are ready to spend time
and energy, we can slowly arrive at the solution for the nervous system
using the deep principle behind solving a system of linear equations.
Since in neuroscience, we cannot have such equations
or shortcuts, we have to arrive at the solution using the hard
way. Since we cannot create an easy equation in neuroscience, everyone
who tries to understand the derived solution has to take the
same
hard way to
appreciate the solution. Since studies of the brain has specialized and
super-specialized into
a
large number of levels, those who are interested
in understanding how the solution was derived will have to spend time to
understand
the
different fields of these specializations. This is a reality.
Here we will use subsets of disparate findings from the list in Table 2 on the first page of this website. We need to use trial and error methods to reach at the solution. By repeating this approach using different subsets of findings, we are expected to arrive at the same solution, which is expected to be the correct solution. Why do we have this much optimism? The optimism is due to the fact that there can only be one unique solution for the system and since we are using very large numbers of findings from different levels of operation of the system, it must be correct. At this point one may ask the following questions. “What is the problem with already published work in neuroscience?” Research work in neuroscience has been carried out by examining finding only from few levels to reach a solution. This has been the practice since one person can only specialize in a few levels of studies and journals have space limitations for articles. “What is the problem with already published work in neuroscience that explains synaptic plasticity?” Here, we made an assumption that synaptic connections make changes and it will be responsible for learning-induced changes from which memories are retrieved. This was initially set up not based on any derivations. Now that we have better knowledge of approaching a system that exhibits disparate features at different levels, we are able to derive an operational mechanism. Due to this reason plastic changes anticipated at the synapses become a weak candidate capable of explaining disparate findings from different levels. Reaching the correct solution implies that it can explain findings from all the levels of the systems to such an extent that we will be confident in replicating the mechanism in engineered systems, which should be the gold standard criterion in understanding the system.
We have to use all the findings from different levels of operation of the nervous system and work hard to find the solution that can remain invariable under all the conditions. It is hard; but this is what we have to do to get to the solution. In this approach, we should be ready for the following. 1) Whatever is the solution that can explain all the findings, we should be ready to tentatively accept it and try to verify it further. 2) Always consider the solution as a hypothesis until we use a large number of triangulations to confirm its accuracy. Once we get exhausted and fail to reject the hypothesis, we should be accepting it as further testable hypothesis. 3) Once we agree that there can be no other way that this system can function and is in agreement with all the expected features of an evolved system, then we should be ready to accept it. So, let us begin.
In order to become successful in solving a system, we have to include all the variables within different non-redundant linear equations (findings) of the system. This is a basic principle for success. Ignoring any single variable will not allow us to solve the system. The main function of the nervous system is generation of first-person inner sensation, within it (which we call as “mind”). Therefore, we have to include a variable for first-person inner sensations within the equations (findings) from appropriate levels for solving the system. Findings from the following levels are to be examined. 1) Systems, 2) Behavior, 3) First-person inner sensation, 4) Electrophysiological, 5) Cellular, and 6) Biochemical. By listing major findings from each level and the major constraint that they bring (Table 1), we will be able to derive a solution for the system.
From the above list, we can see a new level – first-person inner sensations - is introduced. This is of paramount importance in the case of the nervous system. Without this, we will not be able to find a mechanism that generates first-person inner sensations. This is the unique property of the nervous system that makes it different from all other systems in the body. So the real challenge is to understand at what locations and by what mechanism does the system generate first-person inner sensations. It will also help us to understand how this function is related to other features of the system – for example behavior. We currently use behavior to study several higher brain functions such as perception and memory and interpret the results. We have been consistently failing to solve the system since we haven’t taken into consideration the variable of first-person inner sensation while solving the system. So here we are using this level and trying to generate equations that contain this variable. What we meant by this in this approach is to define the relationship between findings from different levels with that of the generation of first-person inner sensations. For example, if a drug blocks memory retrieval as evidenced by lack of behavioral motor action indicative of memory retrieval, now we have to consider that the drug is blocking either generation of first-person inner sensation or its connected pathway towards the behavioral motor action. By continuing this approach, we hope to clarify the pathway of generation of inner sensations and its relationship with behavior. We also hope to understand how this pathway is generated during learning so that it can be reactivated at the time of memory retrieval. We should also make sure that the unique solution for the system should be compatible with all the previous experimental observations. All these functions are expected to operate at physiological time-scales of milliseconds. Therefore, we will avoid any delayed operations observed within the system for the purpose of solving the system. Explaining all these will ultimately clarify the mechanism of nervous system functions.
|
Finding |
|
|
| Memories are virtual first-person inner sensations - which can be viewed as hallucinations (a sensory experience of something in its absence). | The system should have an operational mechanism to generate hallucinations. (This was also the view of Marvin Minsky, a pioneer in Artificial Intelligence research (Minsky, 1980). | An operational mechanism should have a specific feature for generating internal sensations (Vadakkan 2007, 2018). |
| A finite system has to generate infinite number of internal sensations. | There should be sharing of unitary mechanisms of operation depending on specific shared features of internal sensations induced. This can be achieved by the combinatorial action of unitary mechanisms of operation. This allows usage of common shared units of internal sensations for shared features of items and events whose memories are retrieved. This increases the efficiency of the system. In conditions that require an infinite number of properties, nature adapts such a mechanism. For example, variations in the light and heavy chain regions of immunoglobulins (Tonegawa, 1983). | Operational mechanism should have a specific feature for generating unitary mechanism for internal sensations. There should be a mechanism that integrates all the units of internal sensations to generate the first-person internal sensation of perception, memory and other higher brain functions (Vadakkan 2016). |
| Associative learning can take place within milliseconds. Memory is retrieved in milliseconds of time. | Learning mechanism should be able to get completed within milliseconds of time. A memory retrieval mechanism should be able to use the learning-induced changes to induce inner sensations of memory within milliseconds of time. |
Learning should take place at physiological time-scales
of milliseconds. A
cue stimulus should be able to induce first-person inner
sensations within milliseconds (Vadakkan 2018).
|
| Higher brain functions can operate only at a narrow range of frequency of oscillating potentials recorded from extracellular matrix space. | The operating mechanism is tightly associated with the vector components that determine the frequency of these oscillations. |
Operational mechanism of both learning and memory
retrieval should be associated with the vector
components of oscillating extracellular potentials
(Vadakkan 2013, 2016, Vaz et al. 2019).
|
| Most learning-induced change will reverse back leading to forgetting. This memory is called working memory. Some of the learning-induced changes will persist for a short period of time responsible for short-term memories. Some changes may persist for long periods of time responsible for long-term memories. | The learning-induced change should be able to explain changes that are responsible for these different types of memories that are classified based on the duration after which they can be retrieved following learning. | It should be possible to demonstrate that most of the learning-induced change is reversible quickly that can then explain the generation of working memory during the short period of time before those changes reverse back. Some of the learning-induced changes should be able to demonstrate mechanisms by which they can continue to persist for both short and long periods of time that can then explain the generation of short-term and long-term memories during the period of time when learning-induced changes persist (Vadakkan 2018). |
| If we can derive a solution that can accommodate all the above constraints, then we are moving in the right direction even though our sensory systems cannot directly sense the first-person inner sensations. If this mechanism can then explain all the remaining features of the system, then it is expected to make predictions. Once the predictions can be verified, we can confirm the mechanism. | ||
Table 1. A list of five unique findings, the constraints offered by them and the necessary feature of the solution. The solution is expected to have all the above four necessary features. A derived solution with all the necessary features can then be examined whether it can explain all the remaining features, such as a) why do the system need sleep? b) what is the explanation for the electrophysiological finding of long-term potentiation and its correlations with memory? When a satisfactory solution is found, it can be further tested to examine whether it can satisfy the constraints offered by all the findings listed in Table 1 on the first page of this web site. Only a correct solution can provide explanations for all these findings.
If we further analyze the constraints, we can see that we have not yet searched for a mechanism that generates inner sensations. Therefore, we can reasonably say that we have to discover a mechanism that is not familiar to us. In this context, the research community should maintain a low threshold for immediately verifying the validity of the arguments in different hypotheses and if found tenable, they should be subjected to further verification.
Are there unitary mechanisms that generate internal sensations?
In systems of the body that need generation of a very large number of outputs (products) using finite resources, these systems use the power of combinatorial effect by using unitary mechanisms. A typical example is the generation of nearly 1011 specific antibodies against the very large number of anticipated antigenic molecules from the environment by a combinatorial mechanism using finite number of variable (V), joining (J) and sometimes diversity (D) gene segments (Tonegawa, 1983; Janeway et al., 2001). This is possible by the common ability of different DNA segments to undergo recombination. Another example is the ability to make very large number of protein molecules using 20 different amino acids. This is achieved by the common properties of amino acids to form peptide bonds on their two ends. Similarly, these amino acids are formed from 4 different nucleotides having the common property to form phospho-diester bonds on their two ends. In summary, whenever a system has to generate very large number of outputs using finite resources, it is most likely that the system has selected a mechanism that utilizes a combinatorial mechanism. Since an infinite number of memories are expected to get generated using a finite number of neuronal processes, it is reasonable to assume that memory is formed from unitary mechanisms and their natural integration is occurring at physiological time-scales. In this context, it is most likely that the system is utilizing unitary mechanisms with the common properties that will allow them to get bonded together. In the case of memory, it is reasonable to expect generation of units of internal sensations that get integrated to generate internal sensation of memory in response to specific cue stimuli. When search is conducted based on the assumption that unitary mechanism operates, it is necessary to find a learning mechanism from which units of internal sensation can be induced and a mechanism that integrates these units to generate internal sensation of memory.
Explain derivation of semblance hypothesis in simple words?
Since the nature of retrieved memory changes as we keep changing the cue stimulus slightly, it indicates that the changes in cue stimulus are capable of inducing specific units of internal sensation. The induction of internal sensation occurring in physiological time scales requires explanation for a feasible cellular mechanism. Since the induced first-person internal sensation is virtual in nature, the aim of the hypothesis building was to examine the system for specific properties and mechanism that can induce such a function. The mechanism should operate by a simple mechanism and should be operating universally to explain similar functions in members of different species of animals. When the hypothesis was developed, care was given to make sure that it fits very well with the constraints offered by a large number of findings given in Table 1 on the front page.
The derivation of the hypothesis has two major stages. Each stage consists of few steps that are numbered.
Stage I
The derivation of the hypothesis has two major stages. Each stage consists of few steps that are numbered. This stage seeks explanations for the changes occurring during associative learning that can be used at a later stage to retrieve first-person internal sensation of memory. This can be approached by different methods. Here, two methods of approach are given. In both methods, few requirements need to be met, which are based on the following assumptions. a) Generation of an infinite number of internal sensations using a finite number of neuronal processes necessitates a combinatorial process occurring from unitary mechanisms, and b) The system should allow binding of the units of internal sensations. For this to occur, there should be a mechanism that holds the structure-function units together (binding property).
Method 1:
1. For the purpose of the derivation of the
hypothesis, memory is viewed as a virtual inner sensation of a sensory
stimulus since the sensory inputs from the item memorized is not present
during the retrieval of memory.
2. We store thousands of memories. A specific
internal or external cue stimulus is required to retrieve a specific
memory.
3. Let us now conduct an imaginary
experiment. Let us look at a yellow-colored pen. While looking at it,
let us assume that a specific set of 105 synapses (out of the
total 1015 synapses in our brain) was activated at different
orders of neurons (1st order being the order close to the sensory
level). If we can specifically stimulate the set of those specific 105
synapses, we can reasonably assume that we are likely to memorize/
visualize that yellow-colored pen.
4. How can we activate a specific set of 105
synapses out of the total 1015 synapses? Saying in a
different way, how can we selectively activate each of those 105
specific synapses from a set of 1015 synapses for retrieving
the memory immediately after associative learning? If we know how we can
activate one of those 105 specific synapses that identify the
item to be memorized, then we can extend the same mechanism to all the
105 synapses.
5. Alternatively, we can address the issue in
a modified way. What is the minimum requirement that satisfies
activation of a synapse? Activation of the postsynaptic terminal
(dendritic spine or spine) can be taken as the equivalent of activating a synapse
since the activation of a postsynaptic terminal takes place after the arrival of
an action potential at its presynaptic terminal.
6.
Since there is no sensory stimulus available from the item to be
memorized, we cannot anticipate any action potential reaching at the
presynaptic terminals of the routes through which it is supposed to
propagate. Therefore, we need to activate the postsynaptic terminals of
the synapses through which stimulus from the item (whose memory is to get retrieved)
had passed before. This should occur in the absence of
arrival of any action potentials at those presynaptic
terminals during memory retrieval.
8. The above arguments get further support from the fact that lateral entry of activity of certain areas of the brain either artificially or by pathological conditions can induce virtual internal sensations in the form of hallucinations with a compelling sense of reality (Selimbeyoglu and Parvizi, 2010).
9. At this point, we come across with two
key questions. 1) Can we activate the postsynaptic terminal of a synapse
in the absence of the arrival of an action potential at the presynaptic
terminal? 2) How can we choose to activate those 105 specific
postsynaptic terminals from the total 1015 synapses for
specific activation immediately after associative learning? What we have
is a specific cue stimulus that activates a specific set of
synapses. We can now arrive at a simple question at the synaptic level:
“How can we activate a specific set of 105 postsynaptic
terminals that would otherwise be activated by the item whose memory is
to be retrieved in the presence of the activation of the specific set of
synapses by the cue stimulus?
10. Let us assume that the cue stimulus
evokes activation (depolarization) of the postsynaptic terminals through
which activity from the learned item pass through. Then, it is
reasonable to argue that some of the synapses through which activity
spreads from the cue stimulus should be physically close enough to some
of the postsynaptic terminals through which activity from the learned
item passed through at the time of learning.
At the time of memory retrieval, a mechanism should exist that can cause
the spread of activity from the
synapses of the cue stimulus to the postsynaptic terminals of the item
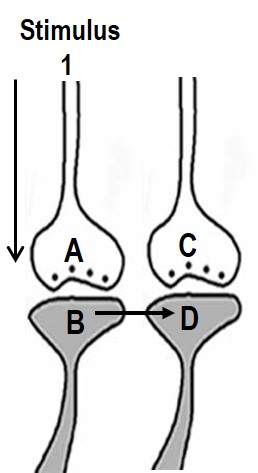
whose memories are retrieved (Fig.1).
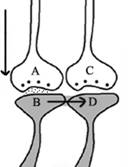
Figure 1.
Illustration of the hypothesized depolarization spread during retrieval
of memory.
During retrieval, the cue stimulus reaching presynaptic terminal A
depolarizes its postsynaptic membrane B, and the depolarization spreads
to postsynaptic membrane D. This can only happen, provided there is a
functional LINK between the postsynaptic terminals B and D. Therefore,
we can assume that a functional LINK is required to be formed between
postsynaptic terminals B and D during learning.
Method 2:
1. Let us imagine that two sensory stimuli, namely stimulus 1 and stimulus 2 undergoes associative learning. At a later time when stimulus 1 (cue stimulus) arrives, it is expected to induce the internal sensation of memory of the second stimulus 2. For this to happen, it is necessary that some changes should occur at the locations of convergence of stimulus 1 and stimulus 2 at the time of learning. (Note that hippocampus known as an area of the brain associated with learning and memory receives inputs from all the different sensory modalities after 3 to 5 orders of neurons from the sensory receptor level). Now, let us examine what changes should be occurring at the location of convergence between two sensory stimuli at the time of learning. What should be the critical change occurring during learning between the synapses activated by the stimulus 1 and stimulus 2? Between what locations of the synapses that these changes should take place? The interaction should take place between those sub-synaptic locations that will enable retrieval of memory of the second stimulus when the first stimulus arrives and vice versa. In this regard, interaction taking place between the postsynaptic terminals of the stimulus 1 and stimulus 2 is suitable. (This was arrived by examining different sub-synaptic areas to find properties that endow them to generate units of internal sensation by trial and error method. This is described in section II). The interaction between the postsynaptic terminals was named as inter-postsynaptic functional LINK (Fig.2). The term "functional" is used to indicate that the formation of the LINK is a function of the activities arriving at the postsynaptic terminals activated by the stimulus 1 and stimulus 2 during associative learning. At the time of memory retrieval, reactivation of the inter-postsynaptic functional LINK is a function of arrival of activity, from either stimuli, at one of their corresponding postsynaptic terminals. The term LINK is written in capital letters to indicate that it is the key element of the hypothesis.
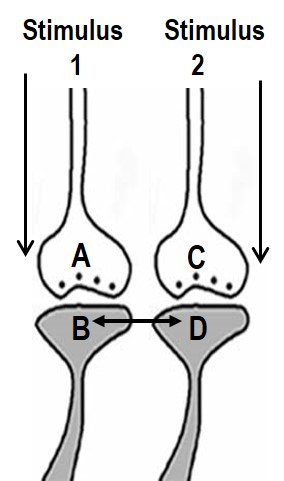
Figure 2. Ihe ilustration shows the formation of hypothesized functional LINK between the two postsynaptic membranes B and D during associative learning between stimulus 1 and stimulus 2.
Different types of
inter-postsynaptic functional LINKs formed during associative learning
The
inter-postsynaptic functional LINKs
formed during associative learning can be of different types:
a.
Those that are formed by removal of water of hydration between the
postsynaptic terminals, which will allow abutting of the membranes. This
requires very high energy and will lead to a rapid reversal of the
functional LINK. This can provide sufficient learning-induced changes
that can last only for a short period of time responsible for working
memory.
b. Strong interaction between the postsynaptic terminals can lead to reversible partial hemifusion between the postsynaptic terminals. This can explain the retention of learning-induced mechanism for more time.
c. Further interaction can lead to reversible complete hemifusion between the postsynaptic terminals that will enable its retention for much more time.
d. If the complete hemifusion can be retained for some time, it is likely that the stabilizing mechanisms can result in long-term maintenance of this.
Inter-LINKing spines are expected to belong to different neurons
At this juncture, it is paramount to understand the origin of the spines that are getting inter-LINKed. According to the studies based on synaptic plasticity thesis, either the spines of a single neuron cluster together at dendritic branches or the synapses at the spines to a single neuron make interactions (Govindarajan et al., 2006; Stuart and Spruston, 2015; Bloss et al., 2018), which are thought to be responsible for the integration of the inputs onto a single neuron.
In contrast to the above hypotheses, the present work approached the problem differently. The inter-LINK formed during associative learning is expected to generate first-person internal sensation at physiological time-scales at the time of memory retrieval. Along with this, it is also expected to generate motor activity corresponding to the retrieved memory. This leads to the question, “To which neuron/neurons should the inter-LINKing spines belong, so that they can maintain specific outputs associated with each of the associatively-learned sensory inputs?” The immediate answer is that they should be belonging to different neurons (Fig.3a). Moreover, since the mean inter-spine distance is even larger than the mean spine diameter (Konur et al., 2003), the inter-LINKing postsynaptic terminals should belong to different neurons. This is expected to be the general rule. There could be exceptions; for example, when axonal terminals of newly formed granule neurons form synapses with a fixed number of dendritic spines of a CA3 neuron (Fig.3b)
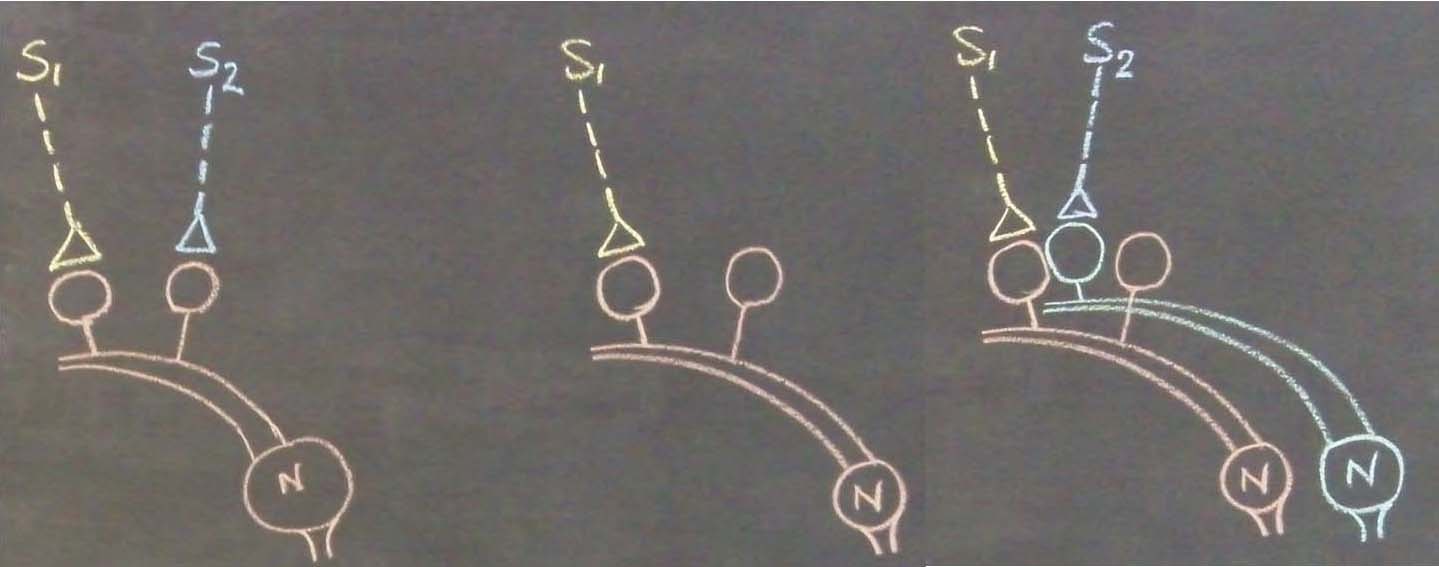
A B C D
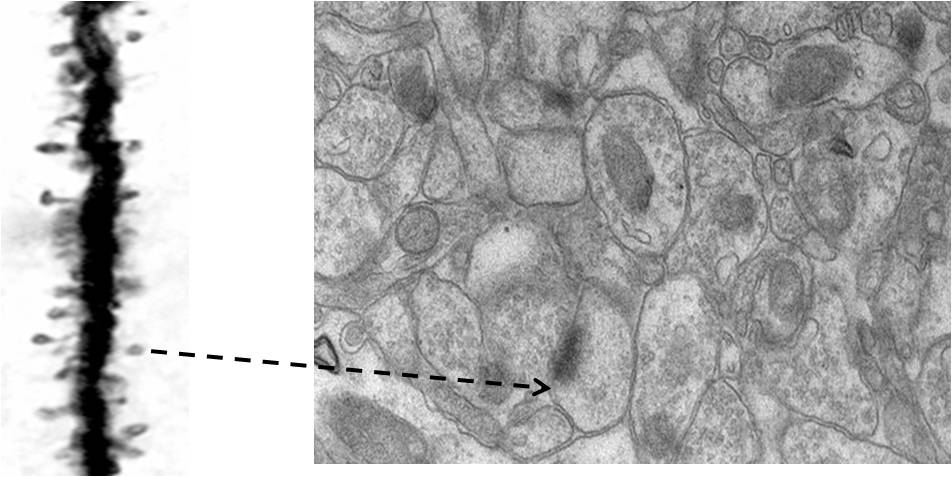
Figure 3b.
Figures showing the importance of making an inference that it is most
probable that the nearest spine to a spine on a dendritic branch of a
neuron is a spine that belong to another dendrite. A) Golgi staining
showing a dendritic branch that has spines on them. These are the inputs
to a neuron. The output terminals of the preceding neurons that synapse
with these spines will not take any Golgi stain. We must assume that
they are present adjacent to them (but both can be seen in electron
microscopic picture shown in Figure B). Nearly any 140 such inputs
arriving at the neuronal axon hillock fire that neuron resulting in
propagation of a signal to all its output terminals. B) An electron
microscopic pictures showing how crowded are the neuronal processes (and
other cells). Extracellular matrix space between neuronal processes (and
glial cells) that we assume to act as an insulating medium preventing
spread of signals between different neurons that have no connections is
very thin. Arrow: Arrow from a spine on figure A is shown towards a
spine in figure B. Note that it has postsynaptic density (PSD) (a dense
dark area) & cellular process adjacent to it is a presynaptic terminal
with synaptic vesicles inside (Please see Figure 9 for more clarity).
Since mean inter-spine distance is more than mean spine diameter (as
seen from figure A), nearest spine to a spine on a dendritic branch is a
spine that belong to another dendrite. If we expect a brain function
through spine-spine interaction and if it needs output from a different
neuron (as demanded by classical conditioning experimental results),
then the nearest spine to a spine on a dendritic branch is a spine that
most probably belonging to a dendrite of another neuron. No scale bars
used.
Inter-postsynaptic functional LINKs can be viewed as biological equivalents of K-lines
K-lines were proposed as the key operational change occurring at the time of associative learning (Minsky, 1980) that is expected to provide the necessary function during memory retrieval. This proposal came as a result of attempts to understand natural intelligence that can be translated into engineered systems.
Stage II
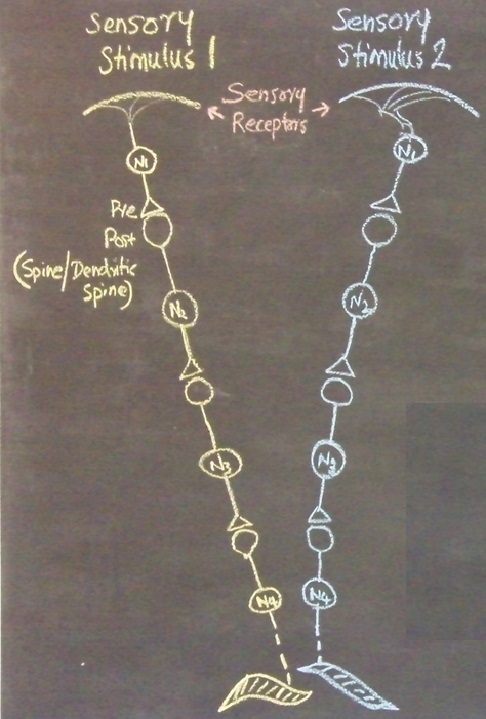
In the next stage, the basic units of semblances occurring at the functionally inter-LINKed postsynaptic terminal are derived. Let us examine the effect of the arrival of the stimulus during memory retrieval. Let stimulus 1 arrive as a cue stimulus (Fig.4). It arrives at the synapse A-B. Postsynaptic potential at B propagates through the inter-postsynaptic functional LINKs and reach towards postsynaptic terminal D. As discussed in Method 1, the arrival of the stimulus 1 (cue stimulus) happens only infrequently. Therefore, when second postsynaptic terminal D is depolarized incidentally in the absence of the arrival of an action potential at its corresponding presynaptic terminal C, then postsynaptic terminal D is expected to get the cellular hallucination that it is receiving sensory inputs through its presynaptic terminal C, resulting in “semblance". This can induce units of virtual inner sensation of memories at the time of memory retrieval and can meet the expectations of a mechanism for memory (Minsky, 1980), if there is a specific operational logic at this location. Before examining the operational logic for the generation of internal sensations, we need to answer two questions. 1) How can a cellular hallucination (semblance) get induced at inter-LINKed postsynaptic terminal D that was previously activated by the item whose memory needs to get retrieved? 2) What is the sensory content of this hallucination?
 b)
b)
.jpg)
Figure 4. a) During retrieval, the cue stimulus reaching presynaptic terminal A depolarizes its postsynaptic membrane B, re-activates the inter-postsynaptic functional LINK. In this manner, depolarization spreads to postsynaptic membrane D evoking cellular hallucination at the postsynaptic terminal D of the arrival of sensory stimuli at its presynaptic terminal C. This is named semblance. b) The propagation of potentials at the synapse A-B and through the inter-postsynaptic LINK B-D provides ionic changes in the extracellular matrix space that contribute vector components for the oscillating extracellular potentials.
The propagation of potentials through the synapse A-B and the IPL B-D provide vector components that are responsible for contributing to the oscillating extracellular potentials (Fig.4b).
When the related learning events continue, one of the postsynaptic
terminals
that already took part in a previous learning event
(either B or D in the
Fig.4) will be used to form functional LINKs
with the postsynaptic
terminals
of the neighboring synapses (seen as additional postsynaptic
terminals
on the right side of the postsynaptic
terminal
D in the left panel, Fig.5). As this process continues, it will
result in the formation of islets of LINKed (LINKable/ re-activatible
during retrieval) postsynaptic
terminals
(right panel, Fig.5).
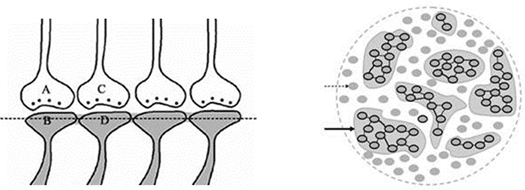
Figure
5.
Left panel: IThe
ilustration
shows
the
formation of islets of
LINKed postsynaptic
terminals.
Continued learning events following the initial learning event can lead
to the formation of multiple
inter-postsynaptic LINKs between the involved
postsynaptic terminals (dendritic
spine heads).
Only two presynaptic
terminals (A and C) and two postsynaptic
terminals
(B and D) are marked. Assume that there are
several
postsynaptic
terminals
arranged in a horizontal plane. The dotted line shows a cross-section
across the
inter-LINKed
postsynaptic
terminals.
Right panel: A hypothetical
cross-sectional view of LINKed postsynaptic terminals of the synapses in
one horizontal plane in a brain region (see the horizontal dotted line
across the postsynaptic membranes in the left panel); in this
illustration, we imagine that all the postsynaptic membranes are in the
same plane. Postsynaptic membranes are shown in small dark circles
(broken arrow). When learning occurs, functional LINKs between activated
postsynaptic terminals can be established. Continued learning using any
of those synapses will increase the number of interconnected
postsynaptic membranes forming islets of functionally LINKed
postsynaptic terminals (solid arrow). Multiple LINKs between the
postsynaptic terminals in an islet can cause spread of postsynaptic potentials across the islet. The individual islets
are expected to be functionally separate from each other.
The
basic units of semblances occurring
at the
functionally inter-LINKed postsynaptic
terminal
are derived (Fig.6).
We need to answer two questions. 1) How can a cellular hallucination
(semblance) get induced at the inter-LINKed postsynaptic terminal D that
was previously activated by the item whose memory needs to get
retrieved? 2) What is the sensory content of this hallucination?
What is the logic behind the generation of cellular hallucination (semblance)?
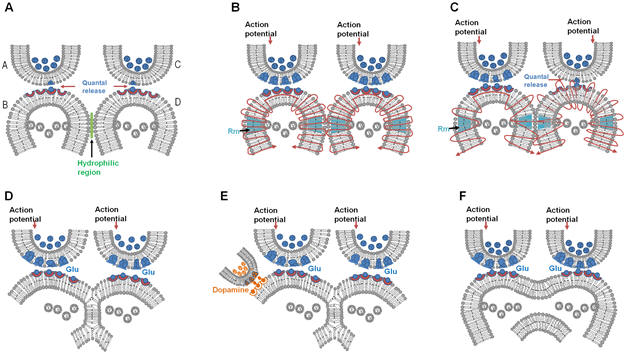
Semblance is the mechanism by which virtual internal sensations are being created. Searching for a cellular location where such a mechanism can be formed resulted in arriving at the requirement for inter-postsynaptic functional LINK. In figure 4, when cue stimulus arrives at the postsynaptic terminal B and re-activates the inter-postsynaptic functional LINK, it activates the postsynaptic terminal D. What makes the postsynaptic terminal to have a cellular hallucination (semblance) that it is receiving activity from its own presynaptic terminal C? The logic can be explained as follows. By default, the postsynaptic terminal D is normally activated by its presynaptic terminal C. To make sure that this is the case, it appears that the Mother Nature has designed an excellent method. There is a continuous quantal release of neurotransmitter molecules from the synaptic vesicles of the presynaptic terminal C even during periods of rest (and sleep). These provide regular arrival of miniature potentials at the postsynaptic terminals. The combined effect of all these potentials is represented by the miniature excitatory postsynaptic potentials (mEPSPs or “minis”). The fact that it is not possible to completely block mEPSPs “even in experimental conditions” indicates that it is a highly conserved default operation of the nervous system. Another necessary condition is the maintenance of oscillatory neuronal activity. The finding that electrical stimulation of the visual cortex produces a visual percept (phosphene) only when high-frequency gamma oscillations are induced in the temporo-parietal junction (Beauchamp et al., 2012) emphasizes the role of oscillating neuronal activity as a system requirement for the semblance formation for creating internal sensations. The lateral spread of activity through the inter-postsynaptic functional LINKs can contribute towards the horizontal component of the oscillating potentials and the synaptic potentials between vertically oriented neurons in the cortex can provide the vertical component. Since inter-postsynaptic spread of potentials occurs perpendicular to the trans-synaptic spread of potentials, this general feature can explain the wave form of oscillating potentials in all other regions in the nervous system, especially where sensory inputs converge.
What is meant by tricking
the inter-LINKed spine to hallucinate?
The inter-LINKed spine heads, like the heads of any other spines (postsynaptic terminals), are continuously being depolarized by quantally-released neurotransmitter molecules all the time, including during sleep. This sets the dominant state of the system that allows any laterally arriving depolarization through the IPL to trick the inter-LINKed spine to hallucinate that it is receiving sensory inputs from the environment through its presynaptic terminal. Are there any real-life examples for the occurrence of such a hallucination? This has been asked recently by few readers. Here are two examples and they highlight the importance of maintaining a dominant state to trick the system to hallucinate.
1. First example is the principle underneath the success of pick
pocketers for successfully doing their job! When I
was in grade six, we had to read from “The Adventures of Tom Sawyer” by Mark
Twain. Young Tom Sawyer along with a group of boys got training to pickpocket. Here, Tom Sawyer had to dissuade the attention of the people by
taking advantage of either introducing alternate sensory stimuli or wait
for their natural occurrence so that he could take a wallet from
someone’s pocket without their attention. Here is a modern version of
pickpocketing (and how to avoid getting pick pocketed!). Watch the events of pick pocketing in this video to see how
a regular background stimulus (stimuli arriving from
regular movements while walking up or down the stairs) can trick someone to think that the stimuli
during pick pocketing is perceived only as a normal stimulus.
Video.
It can be
The above two examples are not perfect. But they can give some good idea
how a system can be tricked to hallucinate, provided you can maintain a
dominant state. Now the question is how can the nervous system end up
operating in this manner? If we look carefully, synapses are having
quantal release from the presynaptic vesicles all the time, which
depolarize the spine heads including that of the inter-LINKed spines.
There are no toxins on the Earth that can completely block this quantal
release (& we hope that we will not bring any toxins from any other
planets or satellites to the Earth!!). In a system where the synaptic
junctions are having continuous quantal release, accidental
coincidence during the early evolutionary stages might have brought two spines
(postsynaptic terminals) to abut each other and form an IPL during
simultaneous arrival of two stimuli from an item. Later, arrival of one
of the stimuli allowed propagation of the postsynaptic potentials to
depolarize the inter-LINKed spine from a lateral direction, tricking
this inter-LINKed spine to hallucinate that it is receiving sensory
inputs from the environment through its presynaptic terminal. This
possibly started providing survival advantage to the animal in instances when the
fastest (light) or first (smell or sound from a curved location where
light cannot curve) arriving stimulus reach the nervous system. This
property continued to get modified over generations to form the
operational mechanism of the nervous systems. This is described in detail in the
paper that explains the evolutionary aspect of the mechanism.
Vadakkan K.I. (2019) A
derived mechanism of the nervous system functions explains aging-related
neurodegeneration as a gradual loss of an evolutionary adaptation Current Aging Science.
Article
Vadakkan K.I. (2016) Substantive nature of
sleep in updating the temporal conditions necessary for inducing units
of internal sensations. Sleep Science.
Article
What is the sensory content of the cellular hallucination (semblance)?
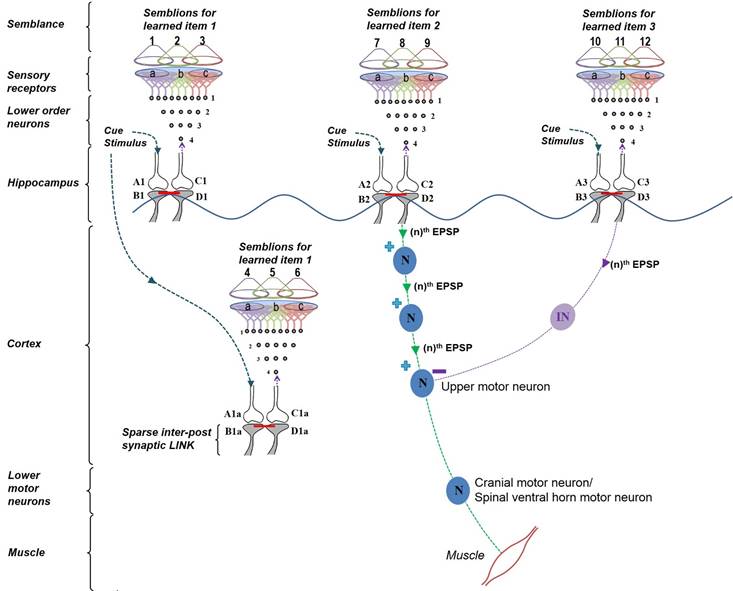
Cue stimulus activates
postsynaptic
terminal
B
that
leads to re-activation of inter-postsynaptic functional LINK and
activates the postsynaptic
terminal
D
that was previously activated by the item whose memory is getting
retrieved now (see Figure 5).
At postsynaptic
terminal
D, this leads to
a
semblance of activity arriving
from
the sensory receptors through
neuron Z. Neuron Z is normally depolarized by activating a set of axonal
terminals of the neurons in order 4 that synapse
to neuron Z’s
dendritic spines (postsynaptic
terminals).
The spatial summation of nearly 40 or the temporal summation of less
than 40 EPSPs (from nearly 40 postsynaptic
terminals
(dendritic spines) out of the nearly
4×104
postsynaptic
terminals
of each neuron) triggers an action potential at neuron Z’s axon hillock
(Note
that the
number of postsynaptic
terminals
(dendritic spines) for a neuron varies.
In the hippocampus,
we expect that the excitatory neurons
have postsynaptic
terminals
in the order of 104).
In the same way, the neurons in set {Y} in
turn receive synaptic transmissions and spread of activity through functional LINKs from a set of neurons {X} in neuronal order 3. By
continuing the extrapolation in a retrograde fashion towards the sensory
level, it will be possible to determine the set of sensory receptors
{SR} whose activation could theoretically cause the activation of
postsynaptic terminal D. Dimensions of internal sensations resulting from the
lateral activation of postsynaptic terminal D can be understood from the
nature of the sensory stimulus that can activate sensory
receptors in the set {SR}. It is likely that activation of subsets of a
minimum number of sensory receptors from {SR} (example, {sr1}, {sr2},
and {sr3} (in Fig.6;
Also see Fig.6 Supplementary) is sufficient to activate
postsynaptic terminal D.
Therefore, a hypothetical packet of minimum sensory stimuli called
“semblion” capable of activating one of the above subsets of sensory
receptors that can activate postsynaptic terminal D is hypothesized as the basic unit of internal
sensation of memory.
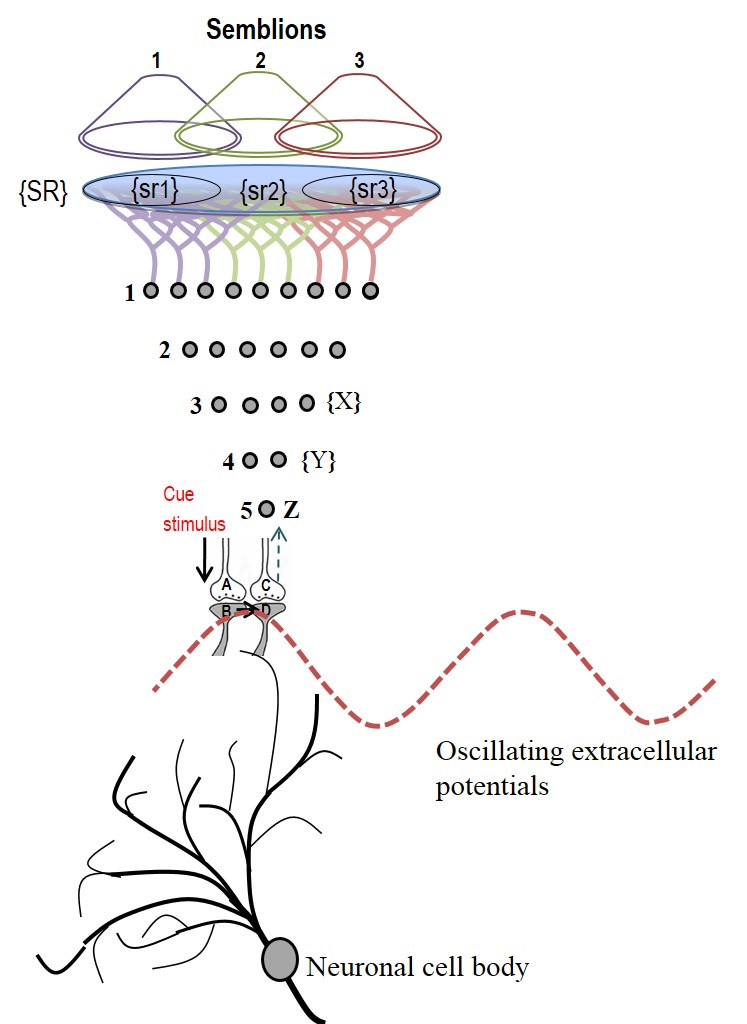
Figure
6.
Schematic representation of sensory elements induced during the
activation of a synapse. The gray circles represent neurons. The numbers
on the left side of the neuronal orders denote their position in
relation to the sensory receptors. Neuron Z is shown in neuronal order
5. During memory retrieval, a cue-stimulus reaching presynaptic terminal
A depolarizes its postsynaptic membrane B and the resulting EPSP at
postsynaptic terminal B re-activates the functional LINK that activates
postsynaptic membrane D. When postsynaptic membrane D is depolarized, it
evokes the cellular hallucination of an action potential reaching its
presynaptic terminal C. This is called synaptic semblance. Note that
presynaptic terminal C belongs to the neuron Z. Either synaptic
semblance occurring at postsynaptic terminal D or random activation of
neuron Z produces the hallucination that it is receiving input from the
set of neurons {Y} that synapse to it. The set of neurons {Y} are
activated by the activation of the set of neurons {X}. The set of
neurons {X} in turn are activated by the set of neurons in the neuronal
order above it. (Recurrent collaterals and projection neurons can also
activate a higher order neuron. For simplicity, these are not shown).
Continuing this extrapolation towards the sensory level identifies a set
of sensory receptors {SR}. It can be seen that stimulation of subsets of
sensory receptor sets {sr1}, {sr2}, and {sr3} from the set {SR} may be
capable of independently activating neuron Z. The dimensions of
hypothetical packets of sensory stimuli capable of activating the
sensory receptor sets {sr1}, {sr2}, and {sr3} are called semblions 1, 2
and 3 respectively. These semblions are viewed as the basic building
blocks of the virtual internal sensations of memory. A cue stimulus can
cause postsynaptic terminal D to hallucinate about any of the semblances
1, 2, 3 or an integral of them. Activation of postsynaptic terminal D by
the cue stimulus can lead to the virtual internal sensation of different
combinations of semblions 1, 2, 3 or an integral of them. The method of
integrating the semblions that match can with the internal sensations
induced by the cue stimulus with that of the item whose memory is
retrieved can be determined by computational studies. Note that the
potentials through the synapse and perpendicularly located IPL
contribute vector components to the oscillating extracellular potentials
(marked by the waveform) (Modified from
Vadakkan, 2011).
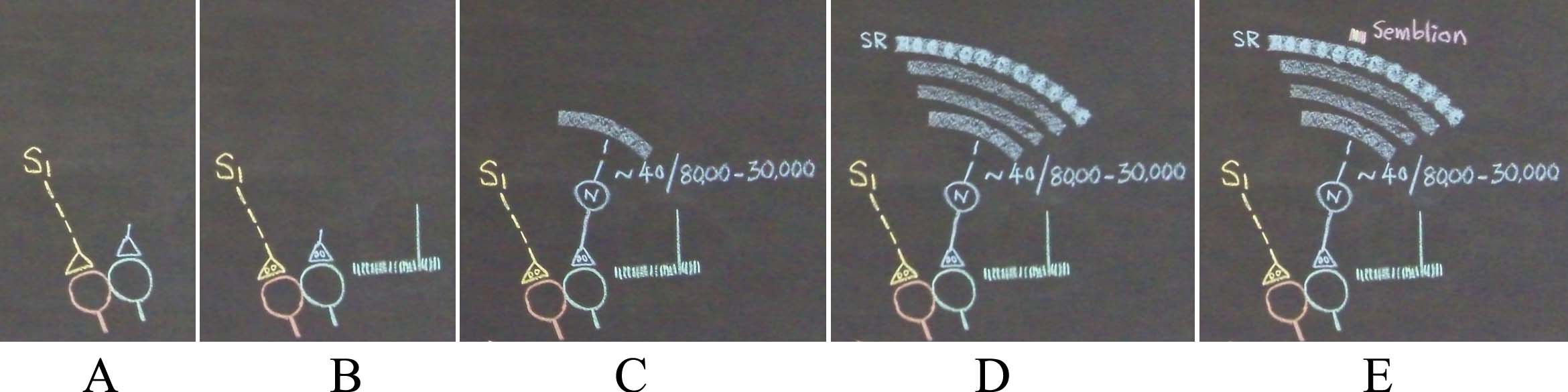
Figure 6 Supplementary. An alternate description is shown in the figure below. A) What can spark a unit of internal sensation when stimulus1 (S1) arrives at one spine of the inter-postsynaptic functional LINK that was formed with the spine of another neuron at the time of learning? The background conditions at the inter-LINKed second spine is that a) the spine head is getting continuously depolarized by the quantal release of neurotransmitter molecules from its presynaptic terminal all the time, which is shown by small vertical lines in the figure, and b) large postsynaptic potential generated by the intermittent arrival of a volley of neurotransmitter molecules when an action potential arrives at its presynaptic terminal (shown by a large vertical line). B) The activation of the inter-LINKed spine from a lateral direction sparks a hallucination that it is receiving a sensory input from the environment through its presynaptic terminal. By making a retrograde extrapolation from the inter-LINKed second spine's presynaptic terminal, we can identify the sensory receptors from where the activity can arrive. Everything here onwards is not associated with neurotransmission. It is virtual in nature. Even though any set of 40 inputs arriving from locations close to the soma (or nearly 140 random inputs arriving from anywhere from the dendritic tree) out of tens of thousands of its inputs (marked in the figure as 8000 - 30,000) can fire the neuron N, the retrograde extrapolation should include all the inputs of neuron N. C) Continuing this process to the level of the sensory receptors identifies a large set of sensory receptors {SR}. From this, a large number of sets of minimum sensory stimuli whose activation can activate subsets of the large sensory receptor set {SR} can be found. D) This extrapolation is continued towards the lower orders of neurons until it reaches the level of the sensory receptors. This will identify all the sensory receptors. The content of the hallucination occurring at the inter-LINKed second spine is about the sensory stimuli stimulating these sensory receptors. Here, we have to think again and ask, "Is it necessary to stimulate all these receptors for an action potential to arrive at the presynaptic terminal of the inter-LINKed spine in real life?" This need not be necessary. In fact, activation of a small subset of these receptors will be able to generate an action potential of the presynaptic terminal's neuron. In other words, content of hallucination at the inter-LINKed spine can be of a sensory stimulus that can activate a fractoin of sensory receptors {SR} that are drawn as round dense areas on the sensory recpetor (SR) layer in the figures D and E. The content of hallucination can be a hypothetical packet of minimum sensory stimuli activating a minimum set of sensory receptors. E) At the top of this picture a set of minimum sensory stimuli that forms the content of the hallucination (internal sensation in the absence of arrival of a sensory stimulus), which is called a "semblion" is shown above one of the senosry receptor subset.
As the cue stimulus passes through different functional LINKs, it evokes a large number of semblances as explained above. Once these possible semblions are identified, their integration can be carried out to obtain a net semblance that matches the sensory characteristics of the item whose memory is retrieved. Attempts to match the different integrational products from the semblions with that of the sensory stimuli from the item whose memories are retrieved will lead to the discovery of the algorithm for neural computations for memory retrieval. The net semblance can exceed more than the threshold without any effect on the retrieved memory. As the functional LINKs get re-activated during memory retrieval, the expected spread of excitatory postsynaptic potential (EPSP) that occurs through some of these functional LINKs can be crucial in adding to the existing sub-threshold EPSP at the axonal hillocks of some neurons that are routinely activated by the oscillatory neuronal activities in the hippocampus and cortex as well as from baseline sensory activities arriving at many neurons. Since the number of functional LINKs continues to change (due to continued associative learning) over the lifespan of the nervous system, the characteristic features of the semblions are also expected to change gradually. This will lead to gradual changes in the net semblances for memory. Related learning can increase the number of LINKed postsynaptic terminals and increase semblance for memory. Absence of retrieval of a specific memory, lack of repetition of learning or lack of related learning will reduce the number of re-activatible inter-postsynaptic functional LINKs and will reduce semblance for retrieval of a specific memory. Along with the induction of semblances, the reactivation of inter-postsynaptic LINKs can also provide additional potentials to the inter-LINKed postsynaptic terminal that can lead to firing of the latter’s neuron if it is kept at a subthreshold activated level (Fig.7).
Figure
7.
Diagram showing the formation of internal sensations and fine control of
the motor activation by a cue stimulus. Oscillating neuronal activity
results in the activation of many downstream neurons. They can be kept
tonically inhibited under resting conditions (not shown) to subthreshold
levels,
such that they can be disinhibited at the arrival of one or a few
excitatory postsynaptic potentials (EPSPs). There were two associative
learning events that occurred previously with the cue stimuli. The first
one was with items 1 and 2. After this first step of associative
learning, the cue stimulus was retrieving memories of items 1 and 2.
Note the reactivation of a sparse inter-postsynaptic functional
What is the nature of inter-postsynaptic
functional LINK?
Different
mechanisms for the formation of inter-postsynaptic LINKs are possible
and are required to explain the formation of internal sensations of other
higher brain functions that operate at different time-scales. These
different types of inter-postsynaptic LINKs with varying half-lives are
suitable to explain perception, working, short- and long-term memories.
A description of some of them is given in Fig.8.
Figure
8.
Different types of reversible inter-postsynaptic functional LINKs. A)
Two abutted synapses A–B
and C–D.
Presynaptic terminals A and C are shown with synaptic vesicles (in blue
color). Postsynaptic terminals (dendritic spines or spines) B and D have
membrane-bound vesicles marked V containing subunits of AMPA receptor
inside them. Action potential arrives at presynaptic terminal A releasing a
volley of neurotransmitters from many synaptic vesicles inducing an
excitatory postsynaptic potential (EPSP) at postsynaptic terminal B.
From the presynaptic terminal C, one vesicle is shown to release its
contents into the synaptic cleft. This quantal release is a continuous
process (even during rest) that leads to the generation of very small
potentials on
postsynaptic membrane D. Note the presence of a hydrophilic region
separating postsynaptic terminals B and D. When an action potential arrives
at presynaptic terminal A, it activates synapse A–B
and generates an EPSP at postsynaptic terminal B. The hydrophilic
region prevents any type of interaction between postsynaptic terminals B
and D. Very high energy is required for excluding the inter-postsynaptic
hydrophilic region (Martens and McMahon 2008). B) Membrane expansion
occurring at physiological time-scales can
provide sufficient energy to exclude the inter-postsynaptic hydrophilic
region, allowing close contact between the postsynaptic membranes in this
region. This forms a transient inter-postsynaptic LINK that lasts only
for a short period of time. During this short period of time, a cue
stimulus-generated action potential arriving at synapse A–B
reactivates this inter-postsynaptic functional LINK and spreads to
postsynaptic terminal D and induces units of internal sensation at the inter-LINKed
postsynaptic terminal D. This can explain working memory. C)
Diagram showing formation of a partial inter-postsynaptic membrane
hemifusion. These vesicles contain glutamate receptor subtype 1 (GluA1).
Activity arriving at the synapse can lead to exocytosis of vesicles
containing AMPA GluA1 receptor-subunits abutted to the cell membranes
and expansion of the postsynaptic membrane at physiological time-scales.
During exocytosis, the vesicle membrane gets incorporated into the
postsynaptic membrane at locations of exocytosis making this region of
the membrane highly re-organizable. This matches with the location where
AMPA receptor subunits were shown to concentrate at the extra-synaptic
locations extending up to 25nm beyond the
synaptic specialization (Jacob and Weinberg 2014). Note the
interaction between the outer layers of membranes of the postsynaptic
terminals. Depending on the lipid membrane composition, the process of close contact between the
membranes described in the above section B) can get converted to a partial
hemifusion state. D) Stage of partial hemifusion can progress to
complete hemifusion. The reversible partial and complete hemifusions are
short-lived and can explain the necessary learning-induced changes
responsible for short-term memory. Some of the hemifusion changes can
get stabilized for different lengths of time. For example, insertion of
a trans-membrane protein across the hemifused segment can maintain the
inter-postsynaptic LINK until this protein gets removed. These changes
can be responsible for long-term memory. E) Dopamine is known to facilitate
motivation-promoted learning. In this diagram dopaminergic input to
spine (postsynaptic terminal) B that results in latter's expansion, which will augment
inter-postsynaptic LINK formation. This can explain the action of
dopamine on learning. Furthermore, it can sustain the hemifused LINK for
a long period of time, which may facilitate its stabilization. F) Hemifusion can advance to a complete fusion state
in pathological conditions and it depends on several factors. Fusion of
the postsynaptic terminals between two different neurons can lead to cytoplasmic
content mixing and cytotoxic cell response. These include dendritic
spine loss and eventually triggering of apoptosis leading to
neurodegenerative changes.
Are
there any experimental
evidence supporting the presence of the inter-postsynaptic functional
LINK?
New technologies are required to test for the
presence of the close contact between the membranes by hydration
exclusion (Figure 8B) in vivo. Another mechanism of inter-postsynaptic
functional LINK is the reversible inter-postsynaptic membrane
hemi-fusion. If this is correct, then examination of the membrane
bilayers at locations where postsynaptic areas are close together is an
opportunity to test the hypothesis. It is also true that at locations
where (sensory) inputs converge, the extracellular matrix space is very
minimal as observed by routing electron microscopic (EM) examination of
these regions. At these locations, abutted postsynaptic membranes are
expected to be seen. However, there are some hurdles. First, the
membrane hemi-fusions are reversible. However, locations within the
hippocampus that has already undergone many associative learning,
stabilization of these hemi-fused areas (most probably by the insertion
of trans-membrane proteins) are expected. Secondly, only a very small
area of membrane hemi-fusion is required for the functional effect of
the formation of inter-postsynaptic functional LINK. Since the area of
the postsynaptic membrane surface that has to be examined for such small
areas of membrane hemi-fusion is very large, dedicated EM studies by
taking serial sections spanning an entire postsynaptic terminal is
required.
Alternatively, examination of a large number of electron microscopic pictures of the hippocampal regions taken for other purposes can be tried. The limitation of this is the lack of resolution of the electron microscopic pictures to visualize the membrane double layer. In a recent EM work (Fig.9) with good resolution, it is possible to observe closely abutted areas, suggesting that they may lack inter-membrane extracellular matrix space. Since dehydration during the tissue processing contribute to these observations, inter-membrane close contacts with hydration exclusion need to be verified using new in vivo techniques. In the above figure, another finding is very striking. There are areas of two layers of hemi-fused membrane for short distances instead of four layers of the two abutting postsynaptic membranes. These are very unlikely to be caused by rotation of the membranes or changes during processing of the tissue. These short spans of reduced number of layers is what is expected by the hemi-fusion process and provide support for the hypothesis until further verifications are carried out. Multiple fused spine heads on a single spine neck seen on dendritic excrescences at the CA3 dendritic tree (Amaral and Dent, 1981; Chicurel and Harris, 1992; Frotscher et al., 1991) is a possible structural modification evolving from long-standing inter-postsynaptic functional LINKs.
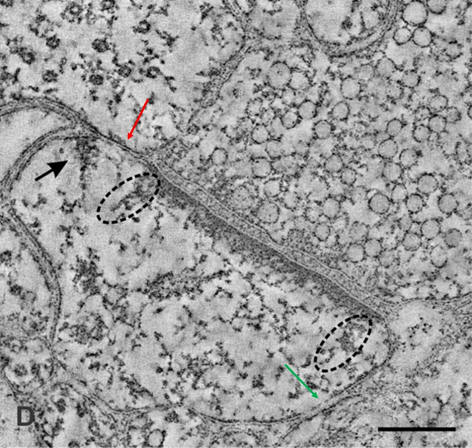
Figure 9. This is figure 4D from Burette A.C, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman M.H, and Weinberg RJ (2012) Electron Tomographic Analysis of Synaptic Ultrastructure. Journal of Comparative Neurology 520 (12): 2697-2711. This figure is modified by inserting one red and another green arrow. The red arrow points towards a likely inter-postsynaptic area with only 2 layers of membrane instead of the expected 4 layers. This is a suspected area of inter-spine hemifusion. Note that that if the structure on which the red line is drawn is a spine, then that spine does not show postsynaptic density in the present section. If it is a spine, then it is reasonable to expect it to have postsynaptic density at the location where it synapses with another presynaptic terminal of possibly another neuron. This will then fulfill the expectations of an inter-neuronal inter-spine hemifusion. The green arrow points to a likely location where the close contact between the membranes is visible, which is likely a location of partial hemifusion. Even though tissue distortions during tissue processing and folded membrane are possibilities, such changes that can span for distances of only 100 nm is very unlikely. This observation needs further dedicated studies for its verification. Furthermore, since some of the cell processes are likely astrocytic pedocytes, dedicated studies are required to verify these observations. Scale bar = 100nm. In contrast to routine electron microscopic sections that use 5 micrometer sections, this study used sections of ~120 nm thickness. This tremendously increased the likelihood of finding suspected hemifused regions of length 100 nm. However, since it is necessary to observe sections along the linear axis of an expected linear structure, the finding of these structural patterns in a random EM section shows a high probability for its universal presence (due to the presence of multiple inter-spine interactions by one spine that leads to islets of inter-LINKed spines - see Fig.5 in FAQs in this page) and warrants further verification.
Why didn't we discover these IPLs until now?
First, there was no reason to search for a mechanism of exclusion of water of hydration at the inter-neuronal inter-spine regions that are activated during associative learning. Secondly, no dedicated studies were carried out to image the lipid bilayers of an entire dendritic spine and its interactions with the abutting spines that belong to different neurons to examine the formation and reversal of inter-postsynaptic functional LINKs by a) exclusion of water of hydration between them, b) formation of inter-spine partial and complete hemifusion, and c) formation of inter-spine fusion in pathological conditions. Since the IPLs are expected to take place in regions of 10nm length, ultra-structural details of entire spine membranes are necessary.
It seems that all the above steps used third-person observations. Where is the examination from a first-person frame of reference?
In Figure 5, the steps needed in finding out the sensory content of the cellular hallucination induced at the postsynaptic terminal D involves examination form a first-person frame of reference. It requires searching backwards from the postsynaptic terminal D towards the sensory receptor level to find out the subset of minimum sensory receptors whose stimulation can activate the postsynaptic terminal D. The minimum sensory stimuli required to activate this subset of sensory receptors constitute the semblion, which is the basic unit of internal sensation. The backward extrapolation from the postsynaptic terminal D towards the sensory receptor level to find out the packets of sensory stimuli is an implicit process taking place during the internal sensations of all the higher brain functions. In this examination, we observe the packets of sensory stimuli (content of the unit of internal sensation) from a first-person frame of reference.
How can we explain long term
potentiation (LTP) in terms of the semblance hypothesis?
For a more detailed description, see published article
The semblance hypothesis was derived to explain plausible synaptic changes occurring during learning suitable for evoking virtual inner sensation of a sensory stimulus during memory retrieval. The operational principle of the formation of semblances resulting in memories is completely different from that of LTP; however, the formation of inter-postsynaptic LINKs can be viewed as a common denominator in both semblance hypothesis and LTP induction (has yet to be confirmed). Explanation of semblance formation through inter-postsynaptic membrane functional LINKs can fill the gaps in our findings of the correlation between memory and LTP and can explain why it has led to a large number of debates. One general argument is that any hypothesis of memory should be able to explain the relationship between LTP and the surrogate behavioral motor activity indicative of memory retrieval.
Previous experiments have shown that spatial learning becomes impaired
after saturation of LTP (Moser et
al., 1998). Later experiments have
shown specific interrelationship between LTP and surrogate markers of
memory retrieval (Whitlock et al., 2006).
In this work it was
shown that one-trial inhibitory avoidance learning in rats produced the
same changes in hippocampal glutamate receptors as the induction of LTP
with high-frequency stimulation. This study showed that learning-induced
synaptic potentiation occludes high-frequency stimulation-induced LTP.
Based on the findings in this work, a plausible
explanation
for the
relationship between LTP and memory through the semblance hypothesis
can be done as follows.
a. Learning first, followed by LTP
induction:
According to the semblance hypothesis, prior
learning events in a caged environment would have already made many
islets of LINKed postsynaptic terminals (dendritic spines) in the
hippocampi of the rats. Since associative
learning opportunities are finite during caged life, we can expect a
slow expansion (by LINKing more postsynaptic terminals with additional
related learning events) of discrete islets of LINKed postsynaptic
terminals as the rats grow up. When rats undergo avoidance learning (a
novel instance of associative learning), we can expect the formation of
functional LINKs between two or more islets of functional LINKs that are
already present in the animal. Even though this is particularly
important in this experimental context, it will also hold true in any
novel associative learning.
In experiments using inhibitory avoidance
testing (Whitlock et al., 2006), not all the recording electrodes
recorded an increase in field excitatory postsynaptic potential (fEPSP)
slope, indicating that ionic changes at the locations of the tips of
these electrodes (CA1 dendritic tree) required to produce an increase in
the fEPSP slope did not take place. However, among those electrodes that
recorded an increase in fEPSP slope after inhibitory avoidance learning,
a sufficient number of Shaffer-CA1 synapses were potentiated. Let I and
II stand for two islets of functionally LINKed postsynaptic terminals
that were already present in the animal before the avoidance learning
session. During learning, it is likely that LINKs were formed between
the islets (islets of LINKed postsynaptic terminals) I and II. This will
generate a sudden increase in the size of an islet of LINKed
postsynaptic terminals to nearly twofold, forming a mega-islet of
LINKed postsynaptic terminals (Fig.10).
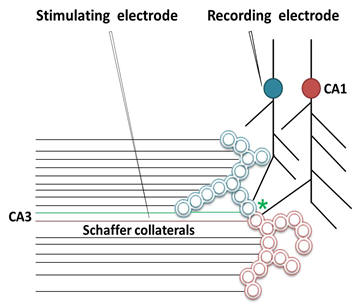
Figure
10.
This illustration explains
the basis of long term potentiation (LTP) based
on the present hypothesis.
The illustration shows potential LINKable site
between islets of
postsynaptic terminals (dendritic spines)
(please see
Figure
5
for details of the islets; they are visualized by
the
hypothetical
cross-sectional view through functionally LINKed postsynaptic
terminals)
that belong to two different CA1 neurons. During an associative
learning, LINK formed between the postsynaptic
terminals
(marked with asterisks) of islets 1 and 2 (large circles) can lead to
the formation of a mega-islet that can continue to contribute to the LTP
recorded from the recording electrode as explained in the text.
The position
of the stimulating electrode is at the Schaffer collaterals. Shaffer
collaterals from the CA3 neurons synapse to the dendritic spines
(postsynaptic
terminals)
of the CA1 neurons. Many of these postsynaptic
terminals
are functionally LINKed to form islets in an animal (see
Figure
5
for details of the islets of functional LINKs). Here two such islets I
and II (large circles) are shown. One of the postsynaptic
terminals
from each of the islets I and II is shown to continue towards the soma
of the CA1 neurons. Activation of any one of the postsynaptic
terminal
within an islet will result in
the
EPSP spread towards the somas of the CA1
neuron. The islets are formed between postsynaptic
terminals
that are concurrently activated during previous associative learning.
During an associative learning of a novel item or during induction of
LTP (note the position of the stimulating electrode is at the Schaffer
collaterals), a new functional LINK may form between the postsynaptic
terminals
(marked asterisks) of islets I and II. This can lead to the formation of
a mega-islet combining the two islets. This can contribute to the LTP
recorded from the recording electrode as explained in the text.
Activation of a postsynaptic
terminal
of this mega-islet of LINKed postsynaptic
terminals
can cause spread of depolarization between its postsynaptic
terminals.
Since a subset of postsynaptic
terminals
in the mega-islet already LINKed to one of the dendritic spines
(postsynaptic membrane) on the dendritic tree of one CA1 neuron,
multiple EPSPs from this subset will reach the main dendrite of a CA1
neuron simultaneously. This results in a summated EPSP at this dendritic
location sufficient to produce a corresponding increase in current sink
in the extracellular matrix. Immediately following the associative
learning event, a proportion of sensory inputs reaching the animal for a
long duration of time is likely to activate the postsynaptic
terminals
of this mega-islet, leading to prolonged activation of the main
dendrites of the above CA1 neuron (until the CA1 neuron begins
homeostatic mechanisms to reduce this prolonged and increased EPSP
generation). The extracellular signal recorded from the apical dendrites
of a population of pyramidal neurons in the stratum radiatum of the CA1
region in response to Schaffer collateral stimulation, namely the
fEPSP, will now show an increase in amplitude and contribute to an
increase in fEPSP slope for a long duration of time (LTP). This
learning-induced LTP can occlude further LTP induction.
b. LTP induction first, followed by learning:
The occlusion process explained in the study
(Whitlock
et al., 2006)
can be considered a bidirectional process, meaning that the induction of
LTP in a sufficient number of synapses that are involved in inhibitory
avoidance learning will prevent consequent avoidance learning. It is
likely that hundreds of axons of the CA3 neurons in the Schaffer
collateral pathway are activated by high-frequency stimulation (LTP
induction), activating the postsynaptic terminals (dendritic spines) of
a CA1 neuron. During this process, many postsynaptic terminals can get
functionally LINKed due to the simultaneous activation of closely placed
postsynaptic terminals by high-frequency stimulation (assuming that
sufficient oxygenation state is present during this process). Some of
these LINKs will occur between the islets of already LINKed postsynaptic
terminals, leading to the generation of mega-islets. Following this, the
activation of one or more postsynaptic terminal by a regular stimulus
(not high frequency) can lead to the spread of depolarization between
the postsynaptic terminals within the mega-islet. Since one or a small
subset of postsynaptic terminals in the mega-islet originates from the
dendritic tree of a single CA1 neuron, multiple EPSPs from these
postsynaptic terminals can reach one dendrite of a CA1 neuron
simultaneously. This results in an increase in the EPSP at these
dendritic locations, leading to LTP. This artificially-induced LTP can
occlude further learning-induced LTP.
If we can artificially induce LTP in a large
number of fibers that includes those that are critical for the learning,
then the animal may not be able to successfully retrieve specific
memories after a new associative learning using those synapses following
the LTP induction. This means that the animal cannot retrieve the
specific memories; i. e., when a cue stimulus tries to retrieve a memory
using these synapses, the induced depolarization spreads across all
those postsynaptic terminals that are LINKed by the LTP induction. The
retrieval using a specific cue now induces synaptic semblances at all
those LINKed postsynaptic terminals in the mega-islet, some of which
were LINKed non-specifically during LTP induction. Activation of
those non-specific postsynaptic terminals will also lead to the
activation of non-specific neurons, leading to the induction of
non-specific network semblances that are not related to the learned
item. In other words, the expected specificity of semblance for the
learned item gets diluted by the large amount of non-specific
semblances, preventing specific memory retrieval.
The following diagram (Fig.11) demonstrates the similarities between the cellular processes in LTP following induction and internal sensation of retrieved memory following associative learning.
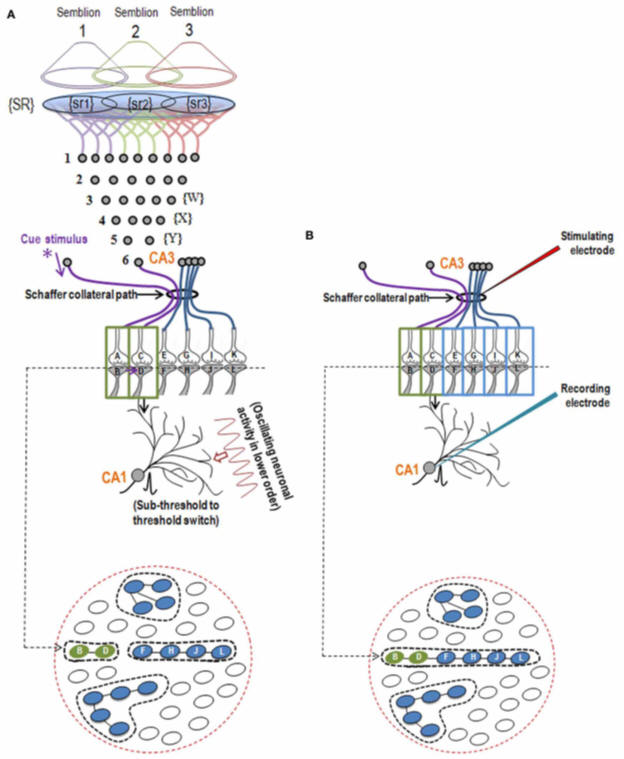
Figure 11. The illustration shows the structural mechanism of formation of internal sensation of memory and its relationship with a possible mechanism of LTP. A) During memory retrieval, a cue-stimulus reaching presynaptic terminal A depolarizes its postsynaptic terminal B, re-activates the hemi-fused inter-postsynaptic membrane and activates postsynaptic terminal D, evoking a cellular hallucination of arrival of sensory inputs at the latter's presynaptic terminal C. In normal conditions, an action potential reaches presynaptic terminal C when the CA3 neuron is activated. The sensory identity of the semblance of activity occurring at the postsynaptic terminal D consists of inputs from the set of neurons {Y} that synapse to the CA3 neuron. The set of neurons {Y} is normally activated by inputs from a set of lower order neurons {X}. The set of neurons {X} in turn is activated by a further large set of its lower order neurons {W}. Continuing this extrapolation toward the sensory level identifies a set of sensory receptors {SR}. {sr1}, {sr2}, and {sr3} are subsets of {SR} and are capable of independently activating the CA3 neuron. Hypothetical packets of sensory stimuli activating sensory receptor sets {sr1}, {sr2}, and {sr3} are called semblions 1, 2, and 3, respectively. The activation of the postsynaptic terminal D by the cue stimulus can lead to the virtual internal sensation of semblions 1, 2, 3 or an integral of them. A CA1 neuron (place cell in the context of spatial memory) is shown receiving sub-threshold excitatory postsynaptic potential (EPSP) from oscillating neuronal activities of its lower order neurons. Cue stimulus-induced activation of postsynaptic terminal D reaches the soma of its neuron in the CA1 region. If the CA1 neuron receives a baseline summated EPSP short of one EPSP to trigger an action potential, then the additional EPSP arriving from the postsynaptic terminal D can add to sub-threshold EPSP, inducing an action potential in the CA1 neuron, resulting in its concurrent activation during memory retrieval; this CA1 neuron will not otherwise be activated in the absence of prior associative learning. This can explain place cell (CA1neuron) firing occurring concurrently with spatial memory retrieval. Bottom Panel: Cross-section through the postsynaptic terminals showing a newly formed functionally LINKed postsynaptic terminals B and D during associative learning. Three other islets are also shown. B) Stimulation of the Schaffer collateral induces LTP by inducing postsynaptic membrane hemi-fusion between postsynaptic terminals that belong to islets of postsynaptic terminals B-D and F-H-J-L forming a mega-islet B-D-F-H-J-L. A regular stimulus at the stimulating electrode has now an increased probability of reaching the recording electrode through the large number of hemi-fused postsynaptic membranes within the large mega-islet, showing a potentiated effect when recorded from the CA1 neuron. Neuronal orders from 1 to 6 are numbered from the sensory receptors. Bottom Panel: Cross-section of an area containing the newly formed mega-islet of functionally LINKed postsynaptic terminals B-D-F-H-J-L formed during LTP induction. Two other islets are also shown. {SR}, Set of sensory receptors; {sr}, subset of sensory receptors. If LTP-induced mega-islets include postsynaptic terminals B and D, it reduces the specificity of retrieved memories in retrieving memories since the spread of activity through different non-specific postsynaptic terminals of the islet induce non-specific semblances (From Vadakkan, 2013).
The hypothesis has used one key assumption that internal sensation is induced at a specific location by a specific mechanism. Why should this be correct?
In order to build a hypothesis, some
assumption has to be made in the beginning.
If one assumption can consistently substantiate all the nervous system
functions, then the probability for that assumption to be correct is
high. This is similar to solving a system of linear equations having a
unique solution. When only one variable remains unknown, then using its
different relations with other variables the value of the unknown
variable can be found mathematically. Alternatively, one is allowed to
assign different values that unknown variable and use trial and error
methods to solve the system. Similarly, in the case of a biological
system where only one variable of internal sensation remains unknown,
large number of known variables and their relationships with the unknown
variable can be used by trial and error methods to solve the system. In
deriving semblance hypothesis, induction of semblances as a system
property was assumed to take place at the inter-LINKed postsynaptic
terminal (dendritic spine) by the reactivation of the inter-postsynaptic
functional LINK due to compelling reasons such as 1) some form of
depolarization is always taking place at the postsynaptic terminal
continuously, 2) the miniature EPSP generation cannot be blocked
completely by any natural or synthetic chemicals on earth, 3) the
formation of the inter-postsynaptic LINK can be achieved as a function
of simultaneous activation of the abutted postsynaptic terminals during
associative learning, 4) induction of semblance can then be derived as a
function of lateral activation of the inter-postsynaptic LINK, 5)
presence of different types of inter-postsynaptic functional LINKs
having different life spans is suitable to explain how changes generated
by learning can last for different durations, 6) it is possible to stabilize the
functional LINK, providing ability to retain ability to retrieve memory
of associatively learned items or events for different duration of time,
7) the lateral spread of activity through the inter-postsynaptic
functional LINK contributes to the horizontal component of the
oscillating potentials which is a requirement for inducing the system
property of internal sensations, 8) semblance is a virtual property that
suits to explain the virtual internal sensations of various higher brain
functions, 9) semblance is a first-person property induced within the
system towards which only the owner of the nervous system has access.
All these fitting conditions make induction of semblance as an
appropriate assumption. The possibilities for a spectrum of changes that
can be formed during the generation of inter-postsynaptic LINK (see
Figure 7) and their maintenance for a wide range of time periods shows
the exact features that one would expect from the basic operational
mechanism. Inter-postsynaptic functional LINK mechanism can operate in
agreement with all the constraints offered by the findings listed in the
Table 1 on the front page of this web site. Due to these reasons, the
hypothesized mechanism is likely to be correct.
Learning and memory were examined due to the
advantages of examining them. Changes can be induced during learning and
these changes are expected to be used to induce memories. Since these
expected changes can be hypothesized, they can be tested to verify the
hypothesis. This led to the derivation of inter-postsynaptic functional
LINK formation and induction of units of internal sensations at the
inter-LINKed spines as the basic operations. It is reasonable to expect
that the basic mechanism of induction of units of internal has shared
properties with the internal sensations of both perception and
consciousness. Slight modification of the process of induction of units
of internal sensations is expected to occur both during perception and
in the operational mechanism of consciousness. In the case of
perception, a real time process of induction of units of internal
sensations has to be explained that can explain the large number of
known properties of perception. This was carried out to explain visual
perception (Vadakkan, 2015c).
In the case of consciousness, it has to explain a) why a large number of
units of internal sensations are getting induced while the animal is at
rest, b) what is the net semblance formed by these units of internal
sensations, and c) how it forms a background matrix upon which internal
sensations in response to specific cue stimuli can be efficiently
induced (Vadakkan, 2010).
What is the basic logic behind
this work?
Let us imagine that there
is a solvable system of linear equations. This means that there are few
equations that contain several variables and these equations form a
system, meaning that they are all interrelated. If we know the values of all
the variables except one, then we will be able to find out the value of
that unknown variable using simple mathematical method. But if we
examine very carefully, we will see that the relationship of the unknown
variable with the known variables within the equations can guide us to
understand what the value of that unknown variable is. The above
relationships are constraints that allow us to understand the value of
the unknown variable. So, instead of using mathematical methods, we can
also find the value of the unknown variable by trial and error method.
In a similar manner,
the nervous system has a very large number of variables that are observed as
different findings at various levels (molecular, cellular,
inter-cellular, electrophysiological, systems, behavioral, and imaging).
We already know all those findings and we have made large number of
correlations between several findings already. Now we have one unknown
variable, which is the generation of internal sensations within the
mind. We are afraid to use it, since we don’t know how it is getting
generated. Let us now use this variable in our findings. Here is an
example. In addition to observing the behavior alone, let us include the
fact that during memory retrieval there is an internal sensation of
memory. Now, internal sensation is the only unknown variable within a
large number of findings within the system. Now, we can use trial and
error methods by using all the constraints offered by findings from
different levels (Given in Table 1 on the front page) to arrive at the
mechanism of generation of internal sensations. The only difficulty is
that we need to examine a very large number of observations from different
levels to arrive at the solution and fine-tune it. Semblance hypothesis has used this method.
For a system that generates first-person internal sensations that are not accessible to third-person observers, it is necessary to use methods used in mathematics and physics to understand a phenomenon that is not sensible to our sensory systems. In this regard, the present work has derived a solution for the system using the underlying principle of finding a solution for a system of linear equations, which is also in agreement with the principle of unification. The resulted solution was used to triangulate (Munafò and Smith, 2018) findings from different levels. The derived solution is now is a position to explain the first-principle behind its operations.
Foremost, present hypothesis has viewed memories in their true sense as first-person internal sensations. The derived solution was found to have background properties for the induction of units of virtual internal sensations. The continuous depolarization of the spine heads (by both quantal release and EPSPs induced by intermittent arrival of action potentials at their presynaptic terminals by sensory stimuli from environment) sets the background state. Associative learning between two stimuli is expected to generate an inter-postsynaptic (inter-spine) LINK (IPL) between the spines that belong to two different neurons. In the above-explained background state, if one of the stimuli (cue stimulus) can incidentally reactivate the IPL, then it is expected to spark a cellular hallucination (semblance) at the inter-LINKed spine of receiving stimulus from the second stimulus. This matches with the expectation of a mechanism for cellular hallucination within the nervous system for explaining memory (Minsky, 1980). This is expected to provide a mechanism for the generation of virtual, first-person internal sensation of memory at physiological time-scales. We can make an extrapolation from the inter-LINKed spine towards the sensory receptors to identify the minimum sensory stimuli required to activate that inter-LINKed spine, which forms units of internal sensation. The combination of a) unique background state of the system, b) possibility for the formation of IPLs with mechanisms that can have different life spans, c) the suitability of the location of IPL that can allow the cue stimulus to reactivate it, d) observation of a suitable mechanism for the induction of virtual first-person internal sensation at physiological time-scales, and e) the perpendicular direction of propagation of potentials through the synapses and IPLs that can contribute vector components to the oscillating extracellular potentials whose frequency within a specific range controls the operation of the system matches with the expectations of a system that generates mind. This coincidence of multiple features provides the most convincing evidence.
Now, if we look at the above mechanism carefully, we can see that the ability to induce cellular hallucination (that constitutes first-person internal sensation) mandates that the dominant state of the system should be that the depolarization of the inter-LINKed spine head occurs with the arrival of activity from its presynaptic terminal. Therefore, lateral activation by the cue stimulus that depolarizes the inter-LINKed spine head from a lateral direction tricking the system to induce cellular hallucination at the inter-LINKed spine that constitutes memory should only occur for a limited duration of time. In other words, when the system keeps the arrival of depolarization from the presynaptic terminal as the dominant feature, then any incidental (or less duration) activation arriving through the IPLs will induce the cellular hallucination. If the ratio of the above two states is high, then the efficiency to trick the system to hallucinate in response to a lateral activation (which constitutes components memory) by a cue stimulus is easy. Since the quantal release that occurs all the time is at a saturated phase, then the system needs to control the duration of lateral activation. After a certain period of time, the system has to enter into a state of sleep (that prevents lateral activations by cue stimuli) following which the system resets itself back to the above dominant state. This provides an explanation for the substantive nature of sleep to the extent that the system will cease to function if it is not allowed to sleep for a few days. This provides another convincing evidence.
The above derived mechanism agrees with all the constraints offered by the very large number of observations from different levels (please refer Table 1 in the front page of this website). Now, some of the convincing evidences that were found during continued examination of this hypothesis are the following. a) Most learning-induced changes will reverse back quickly as the animal moves through the environment, explaining working memory. The proportion of what remains is very less and they remain for varying periods of time for short- and long-term memories. In this regard, we expect that most of the learning-induced mechanism should be able to reverse back quickly. It should leave a small proportion of learning-induced changes to last for varying periods of time. So if we have arrived at the actual mechanism, then we should be able to observe changes to explain the above. By examining Figure 8 in this page, it can be seen that the formation of inter-postsynaptic functional LINKs by exclusion of water of hydration (Fig.8B) requires huge amount of energy and it reverses back quickly. Only a small proportion of these LINKs can form partial and complete hemifusions (Fig.8C,D) and will last for different periods of time. This forms a perfect fit with what we expect from learning-induced changes. This perfect fit shows that this mechanism is inevitable. b) As a continuation of what we found just now, there should be a mechanism for stabilization of learning-induced changes for a long period of time. Since the stage of complete hemifusion (Fig.8D) can be stabilized by different methods, it provides a suitable mechanism. In addition, when the newly formed inter-LINKed spines become part of an islet of inter-LINKed spines, it will be able to get both activated more frequently enabling its long-term maintenance. c) Furthermore, since dopamine cause spine enlargement (Fig.8E), it augments inter-postsynaptic functional LINK formation and its stabilization. This can result in retention of memories for a long period of time. This is another example for a perfect fit. d) Several correlations were found between the ability to learn and induce LTP. The derived mechanism has explained all those correlations and in addition explained some of the remaining uncorrelated observations in the field. This ability of the derived work provides further convincing evidence. e) Ability to provide a framework of a mechanism for perception, explaining various features of visual perception and finding a comparable circuitry for olfactory perception in a remote species Drosophila provides another convincing evidence. f) Ability to provide a framework for internal sensation of consciousness and how anesthetic agents can lead to loss of consciousness provides another evidence. g) Ability to explain a large number of common features of neurodegenerative disorders as a loss of function of the normal operational mechanism is another convincing evidence.
What are the key features of the testable circuit?
Functions of the brain include receiving sensory information, conscious interpretation of some of them, behavioral motor activities in response to them based on previous associative learning, and storing some of the newly received information. What type of a functional map of the nervous system can incorporate these functions? Why have we not succeeded in understanding the nervous system yet? What are we missing here? Once we understand the circuitry, it is necessary to explain a large number of functions within the system at different levels. What type of a circuit map can provide all these different types of observed features within the nervous system?
One of the essential features of the
brain function is the formation of first-person inner sensations of
higher brain functions (e.g. perception, memory, and consciousness) as a first-person property. Studying this property
requires a completely new approach separate from current anatomical,
molecular biological and electro-physiological approaches; but at the
same time adhering to latter's basic principles. In order to understand
what approaches need to be taken, it is required to build a hypothesis
that can explain both first-person internal sensations and third-person
findings from various levels. Since most of the higher brain functions
are first-person internal sensations within the mind, the main emphasis
while searching for the circuit properties should be given to explain
it. In other words, the working hypothesis should have bridges from
cellular and electro-physiological properties to that of the virtual
internal sensation.
Once a hypothesis is built, it
requires to be tested by three essential steps. First, test whether the
hypothesis can explain what we have already discovered in various
faculties of brain sciences. Luckily, the nervous system is complex
enough that we can ask many questions to verify the validity of the
hypothesis. Secondly, the new features in the hypothesis can be tested
and its predictions can be verified. The third step is to address the
issue of the first-person properties of brain functions. We need to
carry out the gold standard test of replicating the mechanism in
engineered systems to confirm the findings. This is necessary since the
higher brain functions of the mind that are first-person properties
require this step to convert internal sensations to appropriate
read-outs so that we experimenters (third-persons) can understand them.
Can we bypass this third step? The only alternative is theoretical. If
the hypothesis can explain a very large number of disparate findings from
multiple levels of the system, then this can be taken as sufficient
evidence.
Semblance hypothesis was proposed
towards achieving the above goals. It has provided explanations for
various electro-physiological, behavioral and systems findings from
different faculties of brain sciences. The essential feature of the
hypothesis is the proposal of inter-postsynaptic functional LINK (IPL).
An examination of the possible nature of this was then carried out. From
examining disease processes that can alter the IPLs, it was possible to
arrive at a reasonable conclusion that IPL is a candidate mechanism.
This can be tested in animals and human samples. Its formation and
functional role can be tested both during associative learning and its
experimental correlate of the induction of long-term potentiation (LTP). By
including IPLs and their functional role, we can obtain a new brain
circuitry, which is explained in the following two figures (Fig.12).
Figure 12. Comparison between the known synaptically-connected circuitry (left side) and the inter-postsynaptic functional LINK-mediated wiring(right side). Left panel: Synaptically connected conventional neuronal circuit diagram. There is one synaptic connection between neurons N1 and N2. The activation of neuron N1 induces an excitatory postsynaptic potential (EPSP) at postsynaptic membrane B. Provided neuron N2 is simultaneously receiving EPSPs from other neurons, the sum of which is just one EPSP short for spatial summation to trigger an action potential, then the EPSP arriving at postsynapse B from the activation of neuron N1 will lead to the firing of neuron N2. The contribution of the EPSP from the activation of Neuron N1 toward the temporal summation of EPSPs to elicit an action potential in neuron N2 should also be considered. Otherwise, a single EPSP or a train of few EPSPs reaching at postsynapse B alone may not induce an action potential of neuron N2. Right panel: Wiring diagram based on the present work. The activation of neuron N1 activates the inter-postsynaptic functional LINKs between the postsynapses in the islet of functional LINKs (see "Frequently asked questions" page on this website). The re-activation of postsynapse B that belongs to neuron N2 can provide EPSP and enable neuron N2 to fire an action potential similar to the threshold conditions explained for neuron N2 of the conventional wiring diagram (in the left panel figure). In addition, EPSPs spread to other hemi-fused postsynapses D, F, H, J, and L (depending on the extent of the spread through the islet) that can reach toward their neuronal somata. According to the supplementary rules, a total of six postsynapses are re-activated here, in comparison to only one by the canonical synaptic transmission (Figure in the left panel). This increases the probability of firing of sub-threshold activated neurons in the next order by bringing them to the threshold for activation. For example, neuron N6 continuously receives (n − 1) EPSPs, just short of one EPSP toward either spatial or temporal summation to elicit an action potential. Arrival of the nth EPSP from the islet of functionally LINKed postsynapses enables neuron N6 to cross the threshold to elicit an action potential (shown in red). If neuron N6 is a motor neuron, it can evoke motor activity concurrent with the re-activation of the functionally LINKed postsynapses B, D, F, H, J, and L. Activity through these LINKed postsynapses will also evoke semblions for the formation of internal sensations provided these are located in regions of oscillatory neuronal activity. All the neurons in red receive sufficient summated EPSPs and fire action potentials. Note that the lateral spread of activity through the inter-postsynaptic functional LINKs provides the horizontal vector for the oscillatory neuronal activities observed both in the cortex and hippocampus. It is marked by a red wave passing through the islet of inter-postsynaptic functional LINKs. Even though ideally it should be drawn over the firing neurons, drawing it over the functional LINKs makes its operation more functionally directed (Modified from
Vadakkan, (2013) A supplementary circuit rule-set for neuronal wiring. Frontiers in Human Neuroscience).The brain activity map that is built of the basic units presented here can explain the large number of nervous system functions (see Table 1 on the first page of this site) that are observed by different faculties of brain science. The basic cellular operating principle for the first person internal sensations of all the higher brain function should be sharing a common cellular mechanism. This will allow the common feature of induction of internal sensations in these higher brain functions. Inter-postsynaptic functional LINKs and the induction of semblance is capable of providing adequate mechanistic cellular features. Since the expected solution that can explain a wide range of findings at several levels is expected to be a unique one, the proposals made by semblance hypothesis can be considered for verification by testing the predictions made by the hypothesis and replicating the mechanism in engineered systems.
Give an example of a
What are the odds of coming up
with a correct hypothesis?
This was a question asked
by one of my colleagues. I realize that this will also be a question
arising in the minds of the readers. From a statistical point of view,
the odds of coming up with a random hypothesis which is correct are very
less. The reality is that I took hypothesis building as a hobby. I
learned how to build a hypothesis and verify whether it can substantiate
nervous system functions from different levels. I used to keep one
hypothesis at a time. When I kept them for sufficient duration, I
started finding weaknesses in them for providing sufficient logical
rigor for a mechanism of nervous system functions. From each failure I
learned a lesson, which helped me to build better ones. Interestingly,
each of my subsequent hypotheses lived longer than the previous ones.
Eventually, I reached my fifth hypothesis, which I kept for nearly a
year before I abandoned it. It was based on charge transfer along the DNA
molecules. It
made sense since neurons do not divide. But it failed in many aspects.
Semblance hypothesis was the sixth hypothesis.
What if
this hypothesis is wrong?
This is a normal feeling following the development of any hypothesis. But if the hypothesis is correct, then the degree of this feeling will reduce with time. This is because there are a large number of ways by which the hypothesis can go wrong and there is only one unique way in which it can be correct. The major task of developing a hypothesis is to figure out the correct solution so that it can explain all the functions observed at various levels. In this approach, there are two major categories of mistakes that can occur and are the following. 1) Let us imagine that the nervous system consists of a large jigsaw puzzle in multiple dimensions and that we are trying to solve it using its parts. The most important thing is that we need to collect all the pieces of the puzzle from multiple levels and bring them on to the table. Since we will only solve for what we have on the table, missing some pieces from few levels can lead to a wrong hypothesis. 2) Secondly, let us imagine that we have brought all the pieces of the puzzle and they are of the same color (can be identified only by their shape, making the puzzle solving more difficult). So, direct matching of the pieces is the only way to confirm whether we are putting the pieces of the puzzle correctly. In this exercise, we may be able to put together a large number of pieces correctly, until we find that the remaining pieces won’t fit into the remaining slots (Hope every one of us had this experience). A good jigsaw puzzle will lead to these situations. Now, we need to dismantle everything and start building it again. Is this hypothesis reaching a stage described as above? Did it bring all the parts onto the table for assembling? Since this hypothesis has incorporated as many pieces of the puzzle as possible from multiple levels (by making sure that no non-redundant findings are left out) and was guided by the tight constraints offered by all those findings (given in Table 1 on the front page), these possibilities are expected to be very less. But, there is still a possibility that it is wrong. This is the reason why there is an open invitation to everyone for falsifying this hypothesis. When we make an observation that cannot be explained in terms of the present hypothesis, then we should consider it wrong. At that time, we should be able to build another hypothesis with more compelling explanations and inter-connectable features. It is also possible that someone can show that the present work is fundamentally wrong. But, it has not happened yet.
What is the motivation behind this work?
1. Nervous system generates first-person internal sensation in the mind, which is virtual in nature. These inner sensations then drive motor outputs in the form of speech and behavior that can be sensed by a third person. Examination of these third person findings alone is not sufficient to understand the system. It will need to acquire knowledge about a) how first-person internal sensations are induced, and b) how they are connected to the motor actions.
2. It is reasonable to expect that there is a solution. The reality is that we have difficulties in understanding it. Our minds can be trained to operate based on Bayes' rule. However, for solving difficult problems, we need to prepare our minds to go against all the priors. We can achieve this only by searching for totally new possibilities by ignoring all the priors. We need to look at the problem afresh similar to an outsider looking into the problem. It is a risky endeavor by all means. First, it will be very difficult to obtain funding to undertake such a work. Secondly, a work done in the absence of funding will be perceived as of low value. Thirdly, it has to face difficulties even from the very stage of publication. Is it worth taking the risk? Since we can use a large number of constraints from our previous findings to guide us towards finding the solution, we have a great opportunity to find the solution. If the constraints can guide us to reach a testable solution, then we should consider this as a potential solution and start taking steps to verify the predictions provided by the solution.
3. Let us imagine that we were able to derive a solution X. This solution should be in agreement with everything that we have already observed. If X can explain all the features of the system at various levels (in principle), then we should be prepared to accept its candidacy as a testable mechanism.
4. The virtual nature of internal sensations
indicates that we should prepare ourselves to arrive at a
first-principle that can provide testable predictions.
Semblance hypothesis has made a testable theoretical finding of a primary neuronal circuitry that resides within the synaptically-connected circuitry and has been evading our attention until now. This is the inter-postsynaptic functional LINK (IPL)-mediated circuitry described in the present work. In addition, the present work also derived a mechanism for the induction of units of internal sensation when IPLs are reactivated. According to the present work, the reactivation of IPLs provides vector components of the oscillating extracellular potentials whose frequency is tightly related to the efficiency of the system to induce internal sensations. An investigation to verify the presence of a spectrum of changes responsible for IPLs and the induction of internal sensations is needed.
What do we need to do in our experiments to verify the present work?
Current experiments are only examining single spines of neurons in to understand higher brain functions (by examining behavioral markers indicative of the generation of internal sensations of those brain functions. We find enlargement of spines and reduction in size or their elimination in experiments. We then start making correlations between these single spine changes with behavior. We fail to examine the interaction between the spine under examination and the spines in the neighboring region that belong to different neurons and how it can influence the internal sensation generated during those higher brain functions. If we look at any electron microscopic picture from cortical regions (for example, in this page see figure 9), the extracellular matrix space between abutted spines is very negligible. Since average inter-spine distance between spines on a dendritic branch is greater than the average spine diameter (Konur et al., 2003), the spines of a neuron are most likely abutted with spines that belong to other neurons. Undertaking studies by looking at how two abutted spines that belong to different neurons are interacting during a higher brain function such as learning at physiological time-scales of milliseconds will facilitate understanding of the inter-postsynaptic LINKs (IPLs) and their functions as explained in the present work. Only changes occurring at matching time-scales of learning or memory retrieval will be relevant. We cannot correlate any late occurring changes with the higher brain functions that generate internal sensations at time-scales of milliseconds.
Why do we need a first-person neuroscience?
Brain is an organ where first-person inner sensations of the mind are being generated. We have no previous experience in dealing with a system that generates first-person properties. It is a real challenge. If it is not for this challenge, many of us who work towards understanding the brain would not have been studying brain in the first place. We like to take these challenges, fail and fail again and eventually we hope one day we will become successful. In this context, we need to be very hard on the problem at hand and we need to face all the realities. Current third-person studies and efforts to use the results of such studies have shown us that we have to find the right tract. A typical example is news that is heartbreaking. During the last ten years, a large number of pharmaceutical companies moved away from drug development for neurological and psychiatric disorders (Wegener and Rujescu, 2013; Burke, 2014; Mehta et al., 2017). What is the reason? It is most likely that they have lost confidence in investing because many of the drug trials failed. For the community, it is a great loss since we have to live without having effective medications either to prevent or to reverse pathological changes in these disorders.
At this juncture, we are forced to ask, “Is there a possible reason why these drug trials constantly fail?” "What do we need to do?" A close examination shows that the studies of the higher brain functions and their dysfunctions are being carried out using surrogate markers such as speech output and behavioral motor actions for making conclusions. We are not attempting to understand the mechanism that operates to generate first-person inner sensations of memory. This is due to the lack of a scientific method to explore the first-person inner sensations of the higher brain functions. In order to design treatment methods to stop the loss of higher brain functions (e. g. memory problems) or alternations of higher brain functions (e.g., hallucinations), an exact science to explain a mechanism by which the first-person internal sensations are getting induced needs to be developed. Fixing the problems will become possible only when we truly understand the normal operational mechanism. In this context, it is necessary to develop specialized methods to explore and understand how first-person internal sensations are induced within the system. This can be best accomplished by having a dedicated first-person neuroscience. The main differences between current third-person neuroscience studies and a future first-person neuroscience studies are given the following table (Table 2).
|
Third-Person Neuroscience |
First-Person Neuroscience |
|
Cellular ch System changes: Oscillating potentials recordable from using either surface or extracellular electrodes. Imaging findings: Changes in signals in fMRI, changes in neuronal ensembles that fire during a higher brain function. Behavioral changes: Speech and motor actions that can provide sensory inputs to third-person experimenters regarding the formation of first-person internal sensations. |
First-person scientific approach deals with studying the mechanism of induction of first-person internal sensations. Currently these are considered as emergent properties. The apparent bottleneck in this approach is the access problem. What we need are methods and tools to overcome the challenges of the access problem. Since third-person experimenters cannot access the first-person properties, the methods to solve the issue involve the following critical steps. 1) Hypothesize a feasible mechanism that explains the nodal points at which internal sensation can emerge and specific conditions. It should have all the elements that can satisfy the requirements to explain findings made at various levels by different fields of neuroscience. 2) The hypothesized mechanism should be able to operate in union with the known circuit properties and should be able to explain various nervous system functions. 3) Using the hypothesized mechanism, develop a circuit to conduct the gold standard test of replicating the mechanism in engineered systems. At this stage, it is required to know the nodal points and conditions in which units of internal sensations emerge as a system property. 4) Devise methods to capture the emergent properties by converting them to suitable readouts for the third-person experimenters. This is a feasible step since we are designing the engineered system. |
Table 2. Key differences between third-person approaches that are being carried out currently and first-person approaches that can be carried out to understand the operation of the nervous system.
What steps are needed to develop
a first-person neuroscience?
How does it change the way that we are studying the nervous system currently?
We have to note that the unique primary function of the nervous system is to generate inner sensations of various higher brain functions. Since the time nervous systems stated sparking inner sensations of memory of the late arriving or non-arriving sensory stimuli (that were previously associated with the fastest or the first arriving cue stimulus when the item was close to the animal) from a remote or hidden item, it started providing a survival advantage to the animals (both the prey and the predator). From that time onwards, for survival purposes, the power of the muscles (of both predators and prey) became secondary. Variations in the mechanism that generates inner sensations are expected to have led to the evolution of related species. Further improvement in the mechanism led to the reasoning and hypotheses building abilities in animals. In this context, the evolution of the nervous system has been taking place primarily to fine-tune the inner sensations for providing a survival advantage to the animals. Based on all these, it is reasonable to view that the circuit features of the black box that generate and integrate inner sensations constitute the PRIMARY circuitry of the nervous system. Hence we need to confirm these findings on an emergent basis, which will direct our efforts to understand the nervous system in the right direction.
How does the nervous system differ from an electronic circuit board with its components?
A printed circuit board (PCB) is a non-conductive plate that electrically connects components through conductive tracks etched on it. In brain, the job of PCB is carried out by the very thin (often negligible) extracellular matrix (ECM) between cellular processes (Fig.13). IPL mechanism shows that during each new learning a new connection is etched between spines that belong to different dendrites at locations of convergence of two sensory stimuli by removing the insulating ECM between them for an area less than 10nm2.
Figure 13. Difference between a printed circuit board (PCB) and brain. The major difference is that in a PCB, electrical paths that connect electronic components are separated from each other by large area of non-conductive (insulating) material. But in the brain, neuronal processes are separated by very thin (& often negligible) insulating medium of extracellular matrix (ECM). Left side: A printed circuit board made of a non-conducting plate on which conductive tracks are etched to connect the circuit components. Note that the surface area of non-conducting plate that does not have the conductive tracks is roughly more than 80% of the surface area of this plate. Right side: An electron microscopic image from the brain cortex. Note that neuronal & glial cell processes occupy most of the surface area with only very negligible insulating ECM space in between them. Note that while acting as an insulting medium, ECM also has two additional functions. 1) Acts as a buffer zone that facilitates ion flux across membranes. 2) Brain functions occur only in a narrow range of frequency of oscillations of potentials within ECM that spans throughout the cortex. It is to be noted that the negligible ECM has to function very faithfully as an insulating medium without causing spread of depolarization to non-targeted neuronal processes. According to the IPL mechanism, the negligible ECM has an added advantage in forming IPLs between abutted spines. Note that even though it may seem easy for forming an IPL between abutted spines, very high energy is required to displace the hydration water between two lipid membranes (Cohen and Melikyan, 2004; Martens and McMahon, 2008). Furthermore, since the repulsive “hydration force” increases steeply when distance between the two bilayers reduces below 20 Å, fusion between two membranes becomes a very high energy requiring process (Rand and Parsegian, 1984; Harrison, 2015). Negligible ECM also emphasizes the importance of maintaining the expected adaptation that prevents conversion of all IPLs formed during life not to progress towards IPL fusion (Vadakkan, 2020).
What does the
thin ECM prompt us to think?
ECM act a buffer zone of ions that
facilitates ion flux across membranes during propagation of
depolarization, which also form part of the oscillating
extracellular potentials that is being maintained in a narrow range
of frequency, which is necessary for all the brain functions. The
most striking feature of ECM in cortex is its thin space occupied by
it (see Fig.13). But the fact that it acts as a robust
insulating medium by virtue of the need for very high energy for
establishing electrical connectivity (Rand
and Parsegian, 1984;
Cohen and Melikyan, 2004; Martens and McMahon, 2008;
Harrison, 2015)
between neuronal processes by displacing fluid ECM between them
offers functional advantage. If learning generates lipid membrane
changes in millisecond time-scales that can overcome the energy
barrier, then establishing inter-neuronal electrical continuity can
form a robust learning mechanism. According to the present work, IPL
mechanism has this advantage.
Furthermore, from studies using artificial membranes
(Leikin, 1987),
it can be inferred that the
area of inter-spine hemifusion is likely to be restricted to
approximately 10nm2
or even less. Astrocytic pedocytes are present only around 50% of
synapses, which too are restricted to only 50% of the perisynaptic
space (Ventura & Harris, 1999).
Except this restriction, the available surface area of ECM where
IPLs can be established is very large. It provides a huge advantage
for the operation of the system.
The high energy requirement for establishing physical interaction
between lipid membranes guarantees that there won't be any non-specific interactions between
neuronal processes that lead to electrical continuity between them.
It also informs us that learning must be triggering a biological
mechanism to overcome the high energy
requirement in millisecond time-scales. Experimental evidence
suggests the role of proteins SNARE and complexin (see
Vadakkan, 2019) in overcoming this high
energy barrier. Details are as follows.
SNARE proteins are known to provide energy for bringing together membranes against repulsive charges and overcome energy barrier related to curvature deformations during hemifusion between abutted membranes (Oelkers et al., 2016). They also generate force to pull together abutted membranes as tightly as possible (Hernandez et al., 2012). By initiating the fusion process by supplying energy (Jahn and Scheller, 2006), SNARE proteins can lead to the formation of characteristic hemifusion intermediates (Lu et al., 2005; Giraudo et al., 2005; Liu et al., 2008). These properties of SNARE proteins highlight their functional significance in forming hemifusion intermediates between lateral spine head regions of spines. Furthermore, protein complexin present within the postsynaptic terminals (Ahmad et al., 2012) is known to interact with the neuronal SNARE core complex to arrest fusion at the stage of hemifusion (Schaub et al., 2006).
Now, the question is "Between which two membranes do the hemifusion occur in the postsynaptic terminals (dendritic spines)?" In the presynaptic terminal there are several synaptic vesicles docked to the membrane that faces the synapse. So, there one can assume that they are hemifused with the membrane of the presynaptic terminal. But in the spines, there are no reports of docked vesicles with its membranes. So the question is "Where do the proteins SNARE and complexin act to generate hemifusion intermediates in the spines?" Detailed electron microscopic & nanometer-scale real time studies are needed here to test for inter-spine interactions that range from mere contacts between membranes to partial and complete hemifusion between membranes of spines that belong to different dendrites. Based on the present work, it is expected that inter-spine interactions occur between abutted spines where sensory stimuli converge during learning (or when they are stimulated together) within millisecond time-scales. Since working memory lasts only for a few seconds, most of them are expected to reverse back. However, since animals have already associatively learned many items and events in the environment, is reasonable to expect several stable hemifused areas between spines at locations of convergence of stimuli. Hence, they can be detected relatively easily. Dendritic excrescences present on the dendrites of CA3 neurons provide hints to this and they can be examined to verify presence of hemifused membranes between spines within them.
When an
animal moves through the environment, it receives several new
associated stimuli. As a result, a large number of IPLs is formed at
locations of convergence of signals from those stimuli. What is most
interesting is that most IPLs will reverse back within a few
seconds. This is expected since majority of our memories of events
during day-to-day life are working memories & last only for a few
seconds.
Since SNARE-mediated vesicle fusion at the presynaptic terminal
takes place within milliseconds, occurrence of a SNARE-mediated IPL formation
of similar time-scales is anticipated.
Solution for the black box problem from the front page of this website
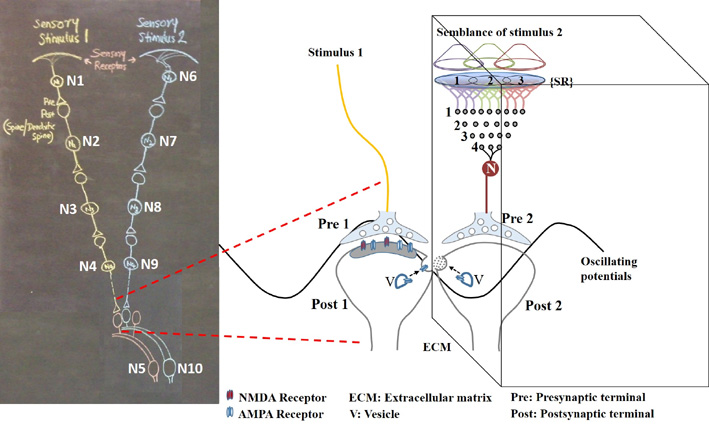
Figure 14.
Figure showing the contents of the black box shown on the front
page of this website
We need to further resolve the dendritic spine structure. This should be carried out without disturbing the integrity of the extracellular matrix volume. This is essential for identifying the formation and reversal of the most common IPLs that are responsible for working memory (see Fig.8B). In order to understand the details of the derived first-principle and the algorithm of integration of units of internal sensations, it is necessary to seek help from basic science fields such as physics, mathematics, computer science, and electronic engineering.
References
Abbas AK, Villers A, Ris L (2015) Temporal phases of long-term potentiation (LTP): myth or fact? Rev. Neurosci. 26(5):507-546. PubMed
Abbott LF (2008) Theoretical neuroscience rising. Neuron 60(3):489-495. PubMed
Ahmad M, Polepalli JS, Goswami D,
Yang X, Kaeser-Woo YJ, Südhof
TC, Malenka RC (2012) Postsynaptic complexin controls AMPA
receptor exocytosis during LTP. Neuron 73, 260–267.
Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 195:51-86. PubMed
Antic SD, Zhou WL, Moore AR, Short SM, Ikonomu KD (2010) The decade of the dendritic NMDA spike. J Neurosci Res 88:2991–3001. PubMed
Beauchamp
MS,
Sun P,
Baum SH,
Tolias AS,
Yoshor D (2012) Electrocorticography links human temporoparietal junction to visual
perception. Nature Neuroscience 15(7)957-959.
PubMed
Burette
AC, Lesperance T, Crum J, Martone M, Volkmann N, Ellisman MH,
Weinberg RJ (2012) Electron Tomographic Analysis of Synaptic
Ultrastructure. Journal of Comparative Neurology 520(12): 2697-2711.
PubMed
Chicurel ME, Harris
KM (1992)
Three-dimensional analysis of the
structure and composition of CA3 branched dendritic spines and their
synaptic relationships with mossy fiber boutons in the rat hippocampus.
J Comp Neurol. 325:169-182.
PubMed
Cohen FS, Melikyan GB (2004) The
energetics of membrane fusion from binding, through hemifusion, pore
formation, and pore enlargement. J. Membr. Biol.
199: 1–14.
Cragg BG (1967) The density of synapses and neurones in the motor and
visual areas of the cerebral cortex.
J Anat 101(Pt 4):639-654.
Edelman S (2012) Six challenges to
theoretical and philosophical psychology. Front Psychol. 3:219.
PubMed
Frotscher ML,
Seress
L,
Schwerdtfeger
WK,
Buhl
E (1991) The
mossy cells of the fascia dentata: a comparative study of their fine
structure and synaptic connections in rodents and primates. J Comp
Neurol. 312:145-163.
PubMed
Gallistel CR, Balsam PD (2014) Time to rethink the neural mechanisms of learning and memory. Neurobiol Learn Mem. 108:136–144. PubMed
Giraudo CG, Hu C, You D, Slovic AM, Mosharov EV, Sulzer D, Melia TJ, Rothman JE (2005) SNAREs can promote complete fusion and hemifusion as alternative outcomes. J. Cell Biol. 170, 249–260. PubMed
Govindarajan A, Kelleher RJ, Tonegawa S (2006) A clustered plasticity
model of long-term memory engrams. Nat Rev Neurosci. 7(7):575-583.
PubMed
Gustafsson B, Wigström H (1990) Long-term potentiation in the hippocampal CA1 region, its induction and early temporal development, Prog. Brain Res. 83:223–232. PubMed
Hangya B, Pi H-J, Kvitsiani D, Ranade SP, Kepecs A (2014) From circuit motifs to computations: mapping the behavioral repertoire of cortical interneurons. Curr. Opin. Neurobiol. 26:117–124. PubMed
Harrison SC. (2015) Viral membrane fusion.
Virology 479-480:498–507.
Hernandez JM, Stein A, Behrmann E, Riedel D,
Cypionka A, Farsi Z, Walla PJ, Raunser S, Jahn R (2012) Membrane fusion
intermediates via directional and full assembly of the SNARE complex.
Science 336(6088):1581–1584.
Jacob AL, Weinberg RJ (2014) The organization of AMPA receptor subunits
at
the postsynaptic membrane. Hippocampus.
25(7):798-812.
PubMed
Jahn R, Scheller RH (2006) SNAREs--engines
for membrane fusion. Nat Rev Mol Cell Biol 7(9):631–643.
Josselyn SA,
Tonegawa S (2020)
Memory
engrams: Recalling the
past and imagining the future.
Science.
367(6473):eaaw4325.
Kennedy MJ, Davison IG, Robinson CG,
Ehlers MD (2020) Syntaxin-4 defines a domain for activity-dependent
exocytosis in dendritic spines. Cell 141:524–35.
Konur S, Rabinowitz D, Fenstermaker VL, Yuste R (2003) Systematic
regulation of spine sizes and densities in pyramidal neurons. J
Neurobiol. 56(2):95-112.
PubMed
Kozlovsky Y, Efrat A, Siegel DP, Kozlov
MM (2004) Stalk phase formation: effects of dehydration and saddle splay
modulus. Biophys J 87(4):2508–21.
Liu T, Wang T,
Chapman ER, Weisshaar JC (2008) Productive hemifusion intermediates in
fast vesicle fusion driven by neuronal SNAREs. Biophys J
94(4):1303–1314.
Lu W, Man H, Ju
W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA
receptors induces membrane insertion of new AMPA receptors and LTP in
cultured hippocampal neurons. Neuron 29(1):243–254.
Lu X, Zhang F,
McNew JA, Shin YK (2005) Membrane fusion induced by neuronal SNAREs
transits through hemifusion. J Biol Chem 280(34):30538–30541.
Martens S, McMahon HT (2008) Mechanisms of membrane fusion: disparate
players and common principles. Nat Rev Mol Cell Biol 9(7):543–556.
PubMed
McMahon
HT, Missler M, Li C, Südhof TC
(1995) Complexins: cytosolic proteins that regulate SNAP receptor
function. Cell 83, 111–119.
Monti MM, Vanhaudenhuyse A, Coleman MR, Boly
M, Pickard JD, Tshibanda L, Owen AM, Laureys S (2010) Willful modulation
of brain activity in disorders of consciousness. N Engl J Med. 362 (7):579-589.
Moser EI, Krobert KA, Moser MB, Morris RG (1998) Impaired spatial learning after saturation of long-term potentiation. Science. 281(5385):2038-2042. PubMed
Munafò MR, Smith GD (2018) Repeating experiments is not enough. Nature 553:399. PubMed
Murayama Y, Biebetamann F, Meinecke FC,
Muller KR, Augath M, Oeltermann A, Logothetis NK (2010) Relationship
between neural and hemodynamic signals during spontaneous activity
studied with temporal kernel CCA. Magn Reson Imaging. 28(8):1095-1103.
PubMed.
Oelkers M, Witt
H, Halder P, Jahn R, Janshoff A (2016) SNARE-mediated membrane fusion
trajectories derived from force-clamp experiments. Proc Natl Acad Sci U
S A 113(46):13051–13056.
Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME (2014)
NMDA spikes enhance action potential generation during sensory input.
Nat Neurosci 17(3):383-390.
Rand RP,
Parsegian VA (1984) Physical force considerations in model and
biological membranes. Can J Biochem Cell Biol 62(8):752–759.
Rumpel S, LeDoux
J, Zador A, Malinow R (2005) Postsynaptic receptor trafficking
underlying a form of associative learning. Science 308(5718):83–88
Rubin DD, Fusi S (2007) Long memory lifetimes require complex synapses and limited sparseness. Front. Comput Neurosci. 1:7. PubMed
Schaub JR, Lu X, Doneske B,
Shin YK, McNew JA. (2006)
Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat.
Struct. Mol. Biol. 13,
748–750.
Schoenbaum G, Chiba AA, Gallagher M (1998)
Orbitofrontal cortex and basolateral
amygdala encode expected outcomes during learning. Nat
Neurosci.1(2):155-159.
Selimbeyoglu A, Parvizi J (2010) Electrical stimulation of the human brain: perceptual and behavioural phenomena reported in the old and new literature. Front. Human Neuroscience 4: 46. PubMed
Shors TJ, Matzel LD (1997) Long-term potentiaion: What's learning got to do with it? Behavioral and brain sciences 20:597-655. PubMed
Spruston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9(3):206-221. PubMed
Stuart GJ, Spruston N
(2015)
Dendritic integration: 60 years of
progress. Nat Neurosci.
18(12):1713-1721.
PubMed
Tonegawa S, Pignatelli M, Roy DS, Ryan TJ (2015) Memory engram storage and retrieval. Curr Opin Neurobiol. 35:101-109. PubMed
Vadakkan KI (2007) Semblance of activity at the shared post-synapses and extracellular matrices - A structure function hypothesis of memory. ISBN:978-0-5954-7002-0 Download
Vadakkan KI (2010) Framework of consciousness
from semblance of activity at functionally LINKed postsynaptic
membranes. Front Psychol. 1:168. doi: 10.3389/fpsyg.2010.00168
Vadakkan KI
(2011) Processing semblances induced through
inter-postsynaptic functional LINKs, presumed biological parallels of
K-lines proposed for building artificial intelligence. Frontiers in
Neuroengineering 4:8.
PubMed
Vadakkan KI (2012a) The nature of "internal sensations" of higher brain
functions may be derived from the design rules for artificial machines
that can produce them. Journal of Biological Engineering. 5;6(1):21.
PubMed
Vadakkan KI (2012b) A structure-function mechanism for schizophrenia.
Frontiers in Psychiatry. 28;3:108.
PubMed
Vadakkan KI
(2013) A supplementary circuit rule-set for the neuronal
wiring. Frontiers in Human Neuroscience. 1;7:170.
PubMed
Vadakkan KI
(2015a) A pressure-reversible cellular mechanism of
general anesthetics capable of altering a possible mechanism for
consciousness. SpringerPlus 4:485
PubMed
Vadakkan KI (2015b) The functional role of
all postsynaptic potentials examined from a first-person frame of
reference. Rev Neurosci. doi: 10.1515/revneuro-2015-0036.
PubMed
Vadakkan KI (2015c) A framework for the
first-person internal sensation of visual perception in mammals and a
comparable circuitry for olfactory perception in Drosophila.
Springerplus. 4:833
Vadakkan KI (2016) The functional role of
all postsynaptic potentials examined from a first-person frame of
reference. Reviews in the Neurosciences (
Vadakkan KI (2018) A learning mechanism completed in milliseconds and capable of transitioning to stabilizable forms can generate working, short and long-term memories - A verifiable mechanism. Peerj Preprints Article
Vadakkan KI (2019)
From cells to sensations: A window to the physics of mind.
Phys Life Rev. 31:44-78.
Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006) Learning induces
long-term potentiation in the hippocampus. Science.
313(5790):1093-1097.
.jpg)